A Method for Calculating Effective Lifetime Risk of Radiation-Induced
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contrast‐Enhanced Ultrasound Bibliography General Principles 1
Contrast‐enhanced Ultrasound Bibliography General Principles 1.Claudon M et al. Guidlines and Good Clinical Practice Recommendations for Contrast Ultrasound (CEUS)‐Update 2008 Ultraschall in Med 2008;29:28‐44 2.Cosgrove. D. Editorial. Eur Radiol Suppl (2004).14[Suppl 8]:1‐3 3.Burns PN, Wilson,S. Microbubble Contrast for Radiological Imaging:1 Principles. Ultrasound Quarterly 2006;22:5‐11 4.Wilson S et al. Contrast‐enhanced Ultrasound: What is the Evidence and What Are the Obstacles? AJR. 2009;193:55‐60 Safety 1.ter Haar GR. Ultrasonic Contrast Agents: Safety Considerations Reviewed. Eur J Radiol 2002;41:217‐221 2. Main MI. Ultrasound Contrast Agent Safety. J Am Coll Cardiol Img 2009:2:1057‐ 1059 3. Pisaglia F et al. SonoVue in Abdominal applications: Retrospective Analysis of 23188 Investigations. Ultrasound in Med and Biol.2006;32:1369‐1375 4. Hynynen K et al. The Threshold for Brain Damage in Rabbits Induced By Bursts of Ultrasound in the Presence of an Ultrasound Contrast Agent (Optison). Ultrasound in Med and Biol;29:473‐481 Voiding Urosonography 1.Darge K. et al. Reflux in Young Patients: Comparison of Voiding US of the Bladder and Retrovesical Space with Echo Enhancement versus Voiding Cystourethrography for Diagnosis. Radiology 1999;210:201‐207 2.Radmayr C et al. Contrast Enhanced Reflux Sonography in Children: A Comparison to Standard Radiological Imaging J Urol 2002;167:1428‐30 Liver Lesion Characterization 1.Bolondi L et al. New Perspectives for the Use of Contrast‐Enhanced Liver Ultrasound in Clinical Practice. Digestive and Liver Disease 2007;39:187‐195 2.Berry J, Sidu, P. -

ACR–SPR Practice Parameter for the Performance of Voiding
The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College is a nonprofit professional society whose primary purposes are to advance the science of radiology, improve radiologic services to the patient, study the socioeconomic aspects of the practice of radiology, and encourage continuing education for radiologists, radiation oncologists, medical physicists, and persons practicing in allied professional fields. The American College of Radiology will periodically define new practice parameters and technical standards for radiologic practice to help advance the science of radiology and to improve the quality of service to patients throughout the United States. Existing practice parameters and technical standards will be reviewed for revision or renewal, as appropriate, on their fifth anniversary or sooner, if indicated. Each practice parameter and technical standard, representing a policy statement by the College, has undergone a thorough consensus process in which it has been subjected to extensive review and approval. The practice parameters and technical standards recognize that the safe and effective use of diagnostic and therapeutic radiology requires specific training, skills, and techniques, as described in each document. Reproduction or modification of the published practice parameter and technical standard by those entities not providing these services is not authorized. Revised 2019 (Resolution 10)* ACR–SPR PRACTICE PARAMETER FOR THE PERFORMANCE OF FLUOROSCOPIC AND SONOGRAPHIC VOIDING CYSTOURETHROGRAPHY IN CHILDREN PREAMBLE This document is an educational tool designed to assist practitioners in providing appropriate radiologic care for patients. Practice Parameters and Technical Standards are not inflexible rules or requirements of practice and are not intended, nor should they be used, to establish a legal standard of care1. -

500 Pyelograms Done After Angiocardiography. the Urinary Tract
9 Section ofRadiology 419 often transitory, it can readily be reproduced by In 200 consecutive pyelograms, analysed both instructing the child to hold back urine and we by congenital heart lesion and urinary tract now believe that this is a common variation of the abnormality, the incidence of abnormal pyelo- normal. It is probably due to distension of the grams was 13 %. The range ofabnormality in both thin-walled proximal urethra, the less distensible is very wide. Pyelogram abnormalities in this bladder neck and distal urethra forming two series and subsequently have included failure of relatively narrow segments. maturation of kidney with pelvis lying intra- renally, solitary kidney, chronic pyelonephritic A 'corkscrew urethra' has been seen in 4 boys kidney, large kidneys, renal rotation, hydro- in this series. It may be associated with reflux. nephrosis, absence of renal pelves, duplication of Cystoscopy and urethroscopy has been normal kidney and ureter (one having a pyelonephritic and in one patient recordings of pressure and lower segment and evidence of vesicoureteric flow were also normal. We can only assume that reflux - Williams 1962), hydroureter and spinal this appearance is produced by redundant folds defects with neurogenic bladder. Factors affecting of mucosa; certainly there has been no evidence cardiac development may affect organogenesis in of obstruction in any of our cases. the urinary tract. The rubella virus was the defined factor in a patient, aged 4 months, with patent Summary ductus arteriosus, pulmonary hypertension and Micturating cystograms carried out in 232 pneumonitis and a miniature left kidney (autopsy children presenting with urinary infection, but proof). -

ACR Manual on Contrast Media
ACR Manual On Contrast Media 2021 ACR Committee on Drugs and Contrast Media Preface 2 ACR Manual on Contrast Media 2021 ACR Committee on Drugs and Contrast Media © Copyright 2021 American College of Radiology ISBN: 978-1-55903-012-0 TABLE OF CONTENTS Topic Page 1. Preface 1 2. Version History 2 3. Introduction 4 4. Patient Selection and Preparation Strategies Before Contrast 5 Medium Administration 5. Fasting Prior to Intravascular Contrast Media Administration 14 6. Safe Injection of Contrast Media 15 7. Extravasation of Contrast Media 18 8. Allergic-Like And Physiologic Reactions to Intravascular 22 Iodinated Contrast Media 9. Contrast Media Warming 29 10. Contrast-Associated Acute Kidney Injury and Contrast 33 Induced Acute Kidney Injury in Adults 11. Metformin 45 12. Contrast Media in Children 48 13. Gastrointestinal (GI) Contrast Media in Adults: Indications and 57 Guidelines 14. ACR–ASNR Position Statement On the Use of Gadolinium 78 Contrast Agents 15. Adverse Reactions To Gadolinium-Based Contrast Media 79 16. Nephrogenic Systemic Fibrosis (NSF) 83 17. Ultrasound Contrast Media 92 18. Treatment of Contrast Reactions 95 19. Administration of Contrast Media to Pregnant or Potentially 97 Pregnant Patients 20. Administration of Contrast Media to Women Who are Breast- 101 Feeding Table 1 – Categories Of Acute Reactions 103 Table 2 – Treatment Of Acute Reactions To Contrast Media In 105 Children Table 3 – Management Of Acute Reactions To Contrast Media In 114 Adults Table 4 – Equipment For Contrast Reaction Kits In Radiology 122 Appendix A – Contrast Media Specifications 124 PREFACE This edition of the ACR Manual on Contrast Media replaces all earlier editions. -

Use of Ultrasound As an Alternative to Fluoroscopy
Use of Ultrasound as an Alternative to Fluoroscopy Rahul Sheth, MD Massachusetts General Hospital, Boston, MA PART 1. INTERVENTIONAL PROCEDURES Fluoroscopy has significantly contributed to the advent and proliferation of image-guided interventions across the gamut of clinical medicine. While these procedures allow for the execution of often complex internal manipulations through a small skin nick rather than a surgical incision, they are not without risk, including the risks of ionizing radiation [1]. In these procedures, fluoroscopy simply serves as an image guidance tool, and as such, alternative imaging modalities that do not rely on ionizing radiation can and should be considered. For example, as a real-time, high resolution imaging modality, ultrasound shares many characteristics with fluoroscopy. However, due to its lack of reliance on ionizing radiation, ultrasound is often the imaging tool of choice for a number of image-guided interventions for which fluoroscopy may also be considered, particularly in the pediatric population. The following examples illustrate specific indications in which ultrasound is preferred over fluoroscopy for interventional procedures. Ultrasound Instead Of Fluoroscopy For Musculoskeletal Procedures Ultrasound is ideally suited for image guided interventions upon the musculoskeletal system [2], as the depth of penetration required for these procedures is typically within several centimeters. A wide array of musculoskeletal interventions can be performed with ultrasound guidance, including arthrocentesis [3,4], joint and soft tissue steroid/anesthetic injections [5,6,7], and aspirations [8,9]. Moreover, while fluoroscopy is the most common imaging modality used for cervical nerve blocks and facet injections, there has recently been tremendous growth in the use of ultrasound for these interventions. -
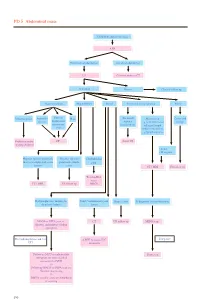
PD 5 Abdominal Mass
PD 5 Abdominal mass Child with abdominal mass AXR No intestinal obstruction Intestinal obstruction US Contrast study or CT Abnormal Normal Clinical follow up Gastrointestinal Hepatobiliary Renal Non-renal retroperitoneal Pelvic Intussusception Appendix Enteric/ Mass (Neonatal) Mass lesion Cystic and abscess duplication/ Adrenal e.g. neuroblastoma/ benign mesenteric haemorrhage enlarged lymph cyst node/ cystic lesion e.g.lymphangioma Reduction under CT Serial US imaging guidance • Solid • Malignant Hepatic/ splenic/ pancreatic Hepatic/ splenic/ Choledochal mass or complicated cystic pancreatic simple cyst lesions cysts CT / MRI US follow up Tc-99m-IDA scan / CT / MRI US follow up MRCP Hydronephrosis / multicystic Solid / complicated cystic Simple cysts If diagnosis is neuroblastoma dysplastic kidney lesion MAG3 or DTPA scan +/- CT US follow up MIBG scan diuretic and indirect voiding cystogram If negative For hydronephrosis and / or ± MRI to assess IVC UTI extension Follow up MCU or radionuclide Bone scan cystogram for more detailed assessment of VUR +/- Follow up MAG3 or DTPA scan for function monitoring +/- DMSA scan for acute pyelonephritis or scarring 190 PD 5 Abdominal mass REMARKS 1 Plain radiograph 1.1 Plain abdominal X-ray (AXR) is useful to exclude intestinal obstruction in children with constipation or abdominal distension, to locate mass, to detect any calcification, and to look for any skeletal involvement. 2 US 2.1 US helps to determine the organ of origin, to define the mass, to look for any metastases and to assess the vascularity of the mass with colour Doppler. A likely diagnosis can usually be made. 3 Nuclear medicine 3.1 Technetium 99m - Mercaptoacetyltriglycine (Tc-99m-MAG3) is the preferred radiotracer for renal scan.1 3.2 Tc-99m-MAG3 renography is able to provide information on renal position, perfusion, differential function and transit times. -

DCMC Emergency Department Radiology Case of the Month
“DOCENDO DECIMUS” VOL 5 NO 5 May 2018 DCMC Emergency Department Radiology Case of the Month These cases have been removed of identifying information. These cases are intended for peer review and educational purposes only. Welcome to the DCMC Emergency Department Radiology Case of the Month! In conjunction with our Pediatric Radiology specialists from ARA, we hope you enjoy these monthly radiological highlights from the case files of the Emergency Department at DCMC. These cases are meant to highlight important chief complaints, cases, and radiology findings that we all encounter every day. Conference Schedule: May 2018 If you enjoy these reviews, we invite you to 2nd - 9: 00 Peds Neuroimaging……Dr Munns, Vezzetti, Leake check out Pediatric Emergency Medicine 10:00 Head Injuries……………….Drs Singh and Kienstra Fellowship Radiology rounds, which are offered 11:00 QI: ED Throughput………….Drs Harrison and Iyer quarterly and are held with the outstanding 9th - 8:00 Bioterrorism………..………Drs Munns and Remick 9:00 Diaster Simulation……………..…Simulation Faculty support of the Pediatric Radiology specialists at Austin Radiologic Association. 16th - 9:00 Environmental Toxins…..…….Drs Fusco and Earp 10:00 Wheezing Beyond Asthma………….……..Dr Allen 11:00 TBD If you have and questions or feedback regarding 12:00 ED Staff Meeting the Case of the Month format, feel free to 23rd - 9:00 Assessing/Enhancing Causality..……Dr Wilkinson 10:00 TBD email Robert Vezzetti, MD at 11:00 TBD [email protected]. 30th - 9:00 M&M……………………..…….Drs Schunk and Gorn 10:00 Board Review: Cardiology………………Dr Ruttan 12:00 Research Update……………….…….Dr Wilkinson This Month: Urinary tract infections are Guest Radiologist: Dr David Leake, MD common in children and generally nothing unusual. -

Voiding Cystourethrography: How I Do It
HK J Paediatr (new series) 2008;13:120-124 Voiding Cystourethrography: How I do it JKF CHAN, JHK NGAN, G LO Abstract Voiding cystourethrography (VCUG) is the most commonly performed fluoroscopic examination for paediatric patients. VCUG however, can be a distressing radiological examination for the patient, parent and medical personnel. We describe our method in performing VCUG and discuss ways in which it could be done safely and effectively. Key words Children; Radiography; Urinary bladder; Vesico-ureteric reflux; Voiding cystourethrography Introduction VCUG can be a distressing radiological examination for the patient, parent and medical personnel. The VCUG is the The voiding cystourethrography (VCUG) is the most most stressful urological investigation performed on commonly performed fluoroscopic examination for children,1 with up to 27% of patients experiencing severe paediatric patients. Properly performed VCUG can provide distress.2 Up to a third of a large cohort of children with useful information on anatomical and functional integrity grade I, II or III reflux were lost to followup, presumably in of the lower urinary tract. It is commonly performed in large part because of the need for repeated VCUG.3 children after urinary tract infection (UTI) or antenally Although other imaging modalities such as radionuclide detected hydronephrosis. It is used in children with cystourethrography (RCU) and voiding sonourography congenital anomalies of renal or lower urinary tract such as (VUS) have been vigorously studied to replace VCUG, none posterior urethral valve (PUV), bladder neck trauma, has the same degree of accuracy and reproducibility. urolithiasis and assessment of unstable bladder; it is the In this article we will discuss how we do VCUG and reference investigation for the study of vesico-ureteric reflux outline ways in which the examination can be done (VUR). -

Radionuclide Techniques for the Detection of Vesicoureteral Reflux
Review Article Radionuclide techniques for the detection of vesicoureteral reflux and their clinical significance Abstract We discuss and try to evaluate the detection of vesicoureteral reux (VUR) by radionuclide techniques and especially direct radionuclide cystography (DRC). Direct radionuclide cystography is applied for more than half a century mainly in children. Vesicoureteral reux has a complex pathology not yet completely under- Christodoulos Likartsis1 MD, BSc stood and is often related to urinary tract infection (UTI) and renal parenchyma scarring that can lead to 2 long-term renal function impairment. Since there is no consensus on the optimal imaging algorithm after Nikoleta Printza MD, PhD 1 the rst febrile urinary tract infection, many imaging strategies have been proposed for VUR detection in Athanasios Notopoulos MD, the last decade, including or not DRC. Views opposing or accepting its use are also presented. MDA, PhD Hell J Nucl Med 2020; 23(2): 180-187 Epub ahead of print: 27 July 2020 Published online: 24 August 2020 Introduction 1. Nuclear Medicine Department, Hippokration Hospital, Thessaloniki, Greece 2. 1st Pediatric Department, Aristotle University, Thessaloniki, bout 30% to 40% of children diagnosed with urinary tract infection (UTI), have Greece primary vesicoureteral reux (VUR) [1]. Children with VUR have 2.6 times higher Aprevalence of renal scarring compared to children without VUR [2]. For most of children with VUR, treatment involves continuous antibiotic prophylaxis (CAP). Mictura- ting cystourethrography (MCU) and direct radionuclide cystography (DRC) are mainly applied for the detection and follow-up of VUR respectively [3] although some authors are not agreeable as for their indications [4, 5]. -

Digest of Council Actions 2020–2021
Digest of Council Actions 2020–2021 acr.org AMERICAN COLLEGE OF RADIOLOGY DIGEST OF COUNCIL ACTIONS 2011-2020 Table of Contents SECTION I ORGANIZATIONAL AND OPERATIONAL POLICIES .......................................... 1 A. GENERAL ......................................................................................................................................... 2 1. ACR Distinguished Achievement Award ................................................................................. 2 2. Honoring the Texas Radiological Society on their Centennial Meeting ................................... 2 3. Honoring the Chicago Radiological Society on their Centennial Meeting ............................... 2 4. Honoring JACR Tenth Anniversary .......................................................................................... 2 5. Honoring the Massachusetts Radiological Society on their Golden Anniversary ..................... 2 6. Council Consultation Regarding Finances ................................................................................ 2 7. Greater Involvement of Young Professionals in the ACR ........................................................ 3 8. Advocacy on Behalf of Radiology ............................................................................................ 3 9. ACR Advocacy Networks ......................................................................................................... 3 10. Strategic Plan ............................................................................................................................ -

Investigation of Urinary Tract Infection
Archives of Disease in Childhood, 1986, 61, 1155-1158 Investigation of urinary tract infection Urinary tract infection (UTI) is common in infants Kingdom recommend a slightly less invasive and children, with a female preponderance except approach, in which all children are investigated by during early infancy. In an early report from urography (or, in appropriately equipped centres, University College Hospital, London, Smellie et al abdominal ultrasonography supplemented when described 200 consecutively diagnosed children with necessary by a renal radionuclide scan), micturating UTI, of whom 188 were investigated by excretory cystourethrography being reserved for children urography or micturating (voiding) cystourethro- under 5 and patients in whom the urogram is graphy, or both.' Abnormalities were found in 50% abnormal or who suffer from frequent in- and in 35% of those investigated after their first fections. 11 12 infection (47% of boys and 28% of girls). In When experts disagree, how is the non-specialist particular, chronic atrophic pyelonephritis (also to decide how to investigate the rather small number known as reflux nephropathy) was detected in 13% of children presenting to him with urinary infection of the total; nearly all of these were children with (two a year for the average general practitioner'3)? bacteriologically proven or clinically suspected mul- To answer this question, it is necessary to under- tiple infections. Vesicoureteric reflux was present in stand the capabilities and, perhaps more import- 30% of investigated subjects but in 85% of those antly, the limitations of the various techniques with pyelonephritis. available for imaging the urinary tract, so that the Numerous subsequent studies have yielded results most appropriate test or combination of tests can be in broad agreement with these, the proportion of selected to suit the needs of each particular case. -

Prevalence of Renal Anomalies After Urinary Tract Infections in Hospitalized Infants Less Than 2 Months of Age
Journal of Perinatology (2010) 30, 281–285 r 2010 Nature Publishing Group All rights reserved. 0743-8346/10 $32 www.nature.com/jp ORIGINAL ARTICLE Prevalence of renal anomalies after urinary tract infections in hospitalized infants less than 2 months of age L Nowell1, C Moran2, PB Smith2,3, P Seed2, BD Alexander3,4, CM Cotten2, JS Wiener2,5 and DK Benjamin Jr2,3 1Duke University School of Medicine, Durham, NC, USA; 2Department of Pediatrics, Duke University Medical Center, Durham, NC, USA; 3Duke University Clinical Research Institute, Durham, NC, USA; 4Department of Medicine and Pathology, Duke University Medical Center, Durham, NC, USA and 5Department of Surgery, Duke University Medical Center, Durham, NC, USA to 24 months of age.7 However, recent data suggest that VUR may Objective: Our aim was to determine the incidence of anatomical not be an independent predictor of recurrent UTIs or new renal abnormalities after a urinary tract infection (UTI) in infants <2 months of scarring.8 A recent randomized controlled trial showed that age hospitalized in the neonatal intensive care unit (NICU). mild–moderate reflux did not increase the incidence of recurrent 6 Study Design: This was a retrospective, single-center cohort study of UTIs and scarring in children. infants <2 months of age in the NICU with a UTI and documented renal Although there are no current recommendations for infants <2 imaging. months of age, the current practice at the Duke University Medical Center, like many medical centers, has been to extend the Result: We identified 141 infants with UTIs. The mean gestational age radiographic recommendations to infants <2 months of age and and birth weight were 28 weeks and 1254 g, respectively.