Masticatory Muscles and the Skull: a Comparative Perspective
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
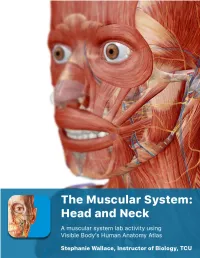
The Muscular System Views
1 PRE-LAB EXERCISES Before coming to lab, get familiar with a few muscle groups we’ll be exploring during lab. Using Visible Body’s Human Anatomy Atlas, go to the Views section. Under Systems, scroll down to the Muscular System views. Select the view Expression and find the following muscles. When you select a muscle, note the book icon in the content box. Selecting this icon allows you to read the muscle’s definition. 1. Occipitofrontalis (epicranius) 2. Orbicularis oculi 3. Orbicularis oris 4. Nasalis 5. Zygomaticus major Return to Muscular System views, select the view Head Rotation and find the following muscles. 1. Sternocleidomastoid 2. Scalene group (anterior, middle, posterior) 2 IN-LAB EXERCISES Use the following modules to guide your exploration of the head and neck region of the muscular system. As you explore the modules, locate the muscles on any charts, models, or specimen available. Please note that these muscles act on the head and neck – those that are located in the neck but act on the back are in a separate section. When reviewing the action of a muscle, it will be helpful to think about where the muscle is located and where the insertion is. Muscle physiology requires that a muscle will “pull” instead of “push” during contraction, and the insertion is the part that will move. Imagine that the muscle is “pulling” on the bone or tissue it is attached to at the insertion. Access 3D views and animated muscle actions in Visible Body’s Human Anatomy Atlas, which will be especially helpful to visualize muscle actions. -

Questions on Human Anatomy
Standard Medical Text-books. ROBERTS’ PRACTICE OF MEDICINE. The Theory and Practice of Medicine. By Frederick T. Roberts, m.d. Third edi- tion. Octavo. Price, cloth, $6.00; leather, $7.00 Recommended at University of Pennsylvania. Long Island College Hospital, Yale and Harvard Colleges, Bishop’s College, Montreal; Uni- versity of Michigan, and over twenty other medical schools. MEIGS & PEPPER ON CHILDREN. A Practical Treatise on Diseases of Children. By J. Forsyth Meigs, m.d., and William Pepper, m.d. 7th edition. 8vo. Price, cloth, $6.00; leather, $7.00 Recommended at thirty-five of the principal medical colleges in the United States, including Bellevue Hospital, New York, University of Pennsylvania, and Long Island College Hospital. BIDDLE’S MATERIA MEDICA. Materia Medica, for the Use of Students and Physicians. By the late Prof. John B Biddle, m.d., Professor of Materia Medica in Jefferson Medical College, Phila- delphia. The Eighth edition. Octavo. Price, cloth, $4.00 Recommended in colleges in all parts of the UnitedStates. BYFORD ON WOMEN. The Diseases and Accidents Incident to Women. By Wm. H. Byford, m.d., Professor of Obstetrics and Diseases of Women and Children in the Chicago Medical College. Third edition, revised. 164 illus. Price, cloth, $5.00; leather, $6.00 “ Being particularly of use where questions of etiology and general treatment are concerned.”—American Journal of Obstetrics. CAZEAUX’S GREAT WORK ON OBSTETRICS. A practical Text-book on Midwifery. The most complete book now before the profession. Sixth edition, illus. Price, cloth, $6.00 ; leather, $7.00 Recommended at nearly fifty medical schools in the United States. -

EDS Awareness in the TMJ Patient
EDS Awareness in the TMJ Patient TMJ and CCI with the EDS Patient “The 50/50” Myofascial Pain Syndrome EDNF, Baltimore, MD August 14,15, 2015 Generation, Diagnosis and Treatment of Head Pain of Musculoskeletal Origin Head pain generated by: • Temporomandibular joint dysfunction • Cervicocranial Instability • Mandibular deviation • Deflection of the Pharyngeal Constrictor Structures Parameters & Observations . The Myofascial Pain Syndrome (MPS) is a description of pain tracking in 200 Ehlers-Danlos patients. Of the 200 patients, 195 were afflicted with this pain referral syndrome pattern. The MPS is in direct association and correlation to Temporomandibular Joint dysfunction and Cervico- Cranial Instability syndromes. Both syndromes are virtually and always correlated. Evaluation of this syndrome was completed after testing was done to rule out complex or life threatening conditions. The Temporomandibular Joint TMJ Dysfunction Symptoms: Deceptively Simple, with Complex Origins 1) Mouth opening, closing with deviation of mandibular condyles. -Menisci that maybe subluxated causing mandibular elevation. -Jaw locking “open” or “closed”. -Inability to “chew”. 2) “Headaches”/”Muscles spasms” (due to decreased vertical height)generated in the temporalis muscle, cheeks areas, under the angle of the jaw. 3) Osseous distortion Pain can be generated in the cheeks, floor of the orbits and/or sinuses due to osseous distortion associated with “bruxism”. TMJ dysfunction cont. (Any of the following motions may produce pain) Pain With: . Limited opening(closed lock): . Less than 33 mm of rotation in either or both joints . Translation- or lack of . Deviations – motion of the mandible to the affected side or none when both joints are affected . Over joint pain with or without motion around or . -

MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients After Whiplash Injury
Journal of Clinical Medicine Article MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury Yeon-Hee Lee 1,* , Kyung Mi Lee 2 and Q-Schick Auh 1 1 Department of Orofacial Pain and Oral Medicine, Kyung Hee University Dental Hospital, #613 Hoegi-dong, Dongdaemun-gu, Seoul 02447, Korea; [email protected] 2 Department of Radiology, Kyung Hee University College of Medicine, Kyung Hee University Hospital, #26 Kyunghee-daero, Dongdaemun-gu, Seoul 02447, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-2-958-9409; Fax: +82-2-968-0588 Abstract: Objective: to investigate the change in volume and signal in the masticatory muscles and temporomandibular joint (TMJ) of patients with temporomandibular disorder (TMD) after whiplash injury, based on magnetic resonance imaging (MRI), and to correlate them with other clinical parameters. Methods: ninety patients (64 women, 26 men; mean age: 39.36 ± 15.40 years), including 45 patients with symptoms of TMD after whiplash injury (wTMD), and 45 age- and sex- matched controls with TMD due to idiopathic causes (iTMD) were included. TMD was diagnosed using the study diagnostic criteria for TMD Axis I, and MRI findings of the TMJ and masticatory muscles were investigated. To evaluate the severity of TMD pain and muscle tenderness, we used a visual analog scale (VAS), palpation index (PI), and neck PI. Results: TMD indexes, including VAS, PI, and neck PI were significantly higher in the wTMD group. In the wTMD group, muscle tenderness was highest in the masseter muscle (71.1%), and muscle tenderness in the temporalis (60.0%), lateral pterygoid muscle (LPM) (22.2%), and medial pterygoid muscle (15.6%) was significantly more frequent than that in the iTMD group (all p < 0.05). -
The Mandibular Nerve - Vc Or VIII by Prof
The Mandibular Nerve - Vc or VIII by Prof. Dr. Imran Qureshi The Mandibular nerve is the third and largest division of the trigeminal nerve. It is a mixed nerve. Its sensory root emerges from the posterior region of the semilunar ganglion and is joined by the motor root of the trigeminal nerve. These two nerve bundles leave the cranial cavity through the foramen ovale and unite immediately to form the trunk of the mixed mandibular nerve that passes into the infratemporal fossa. Here, it runs anterior to the middle meningeal artery and is sandwiched between the superior head of the lateral pterygoid and tensor veli palatini muscles. After a short course during which a meningeal branch to the dura mater, and the nerve to part of the medial pterygoid muscle (and the tensor tympani and tensor veli palatini muscles) are given off, the mandibular trunk divides into a smaller anterior and a larger posterior division. The anterior division receives most of the fibres from the motor root and distributes them to the other muscles of mastication i.e. the lateral pterygoid, medial pterygoid, temporalis and masseter muscles. The nerve to masseter and two deep temporal nerves (anterior and posterior) pass laterally above the medial pterygoid. The nerve to the masseter continues outward through the mandibular notch, while the deep temporal nerves turn upward deep to temporalis for its supply. The sensory fibres that it receives are distributed as the buccal nerve. The 1 | P a g e buccal nerve passes between the medial and lateral pterygoids and passes downward and forward to emerge from under cover of the masseter with the buccal artery. -

Tinnitus and Temporomandibular Joint Disorder Subtypes
TINNITUS AND TEMPOROMANDIBULAR JOINT DISORDER SUBTYPES SUSEE PRIYANKA RAVURI A thesis Submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE IN DENTISTRY University of Washington 2017 Committee Edmond L. Truelove Peggy Lee Lloyd A. Mancl Program Authorized to Offer Degree: Oral Medicine 1 © Copyright 2017 Susee Priyanka Ravuri 2 University of Washington ABSTRACT Tinnitus And Temporomandibular Joint Disorder Subtypes Susee Priyanka Ravuri Edmond L. Truelove B.S., D.D.S., M.S.D. Oral Medicine OBJECTIVE: The purpose of this study was to assess the prevalence of tinnitus within a TMD population and to determine an association between the presence of tinnitus and type of TMD diagnoses. METHODS: A secondary data analysis was performed using data from ‘Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) baseline (Validation project) study and follow up (Impact project) study. Self-reported questionnaires for reporting tinnitus and medical history and gold standard diagnoses after clinical examination were used. Log-binomial regression was used to compute risk ratios for tinnitus by TMD subtype and adjusted for patient characteristics. All statistical analysis was performed using SAS 9.3 software (SAS Institute), and a two-sided significance level of 0.05 to determined statistical significance (p<0.05). RESULTS: At baseline, 614 subjects met required criteria for TMD diagnosis. Prevalence of tinnitus within sample was 41% (253 of 614). Approximately 80% of TMD subjects received a MPD diagnosis. Tinnitus frequency in the MPD group was 48% (238/495) while subjects without MPD diagnosis the rate of tinnitus was 13% (15 of 119). Using log-binomial regression analysis, the risk ratio for tinnitus was calculated. -

New Knowledge Resource for Anatomy Enables Comprehensive Searches of the Literature on the Feeding Muscles of Mammals
RESEARCH ARTICLE Muscle Logic: New Knowledge Resource for Anatomy Enables Comprehensive Searches of the Literature on the Feeding Muscles of Mammals Robert E. Druzinsky1*, James P. Balhoff2, Alfred W. Crompton3, James Done4, Rebecca Z. German5, Melissa A. Haendel6, Anthony Herrel7, Susan W. Herring8, Hilmar Lapp9,10, Paula M. Mabee11, Hans-Michael Muller4, Christopher J. Mungall12, Paul W. Sternberg4,13, a11111 Kimberly Van Auken4, Christopher J. Vinyard5, Susan H. Williams14, Christine E. Wall15 1 Department of Oral Biology, University of Illinois at Chicago, Chicago, Illinois, United States of America, 2 RTI International, Research Triangle Park, North Carolina, United States of America, 3 Organismic and Evolutionary Biology, Harvard University, Cambridge, Massachusetts, United States of America, 4 Division of Biology and Biological Engineering, M/C 156–29, California Institute of Technology, Pasadena, California, United States of America, 5 Department of Anatomy and Neurobiology, Northeast Ohio Medical University, Rootstown, Ohio, United States of America, 6 Oregon Health and Science University, Portland, Oregon, ’ OPEN ACCESS United States of America, 7 Département d Ecologie et de Gestion de la Biodiversité, Museum National d’Histoire Naturelle, Paris, France, 8 University of Washington, Department of Orthodontics, Seattle, Citation: Druzinsky RE, Balhoff JP, Crompton AW, Washington, United States of America, 9 National Evolutionary Synthesis Center, Durham, North Carolina, Done J, German RZ, Haendel MA, et al. (2016) United States of America, 10 Center for Genomic and Computational Biology, Duke University, Durham, Muscle Logic: New Knowledge Resource for North Carolina, United States of America, 11 Department of Biology, University of South Dakota, Vermillion, South Dakota, United States of America, 12 Genomics Division, Lawrence Berkeley National Laboratory, Anatomy Enables Comprehensive Searches of the Berkeley, California, United States of America, 13 Howard Hughes Medical Institute, M/C 156–29, California Literature on the Feeding Muscles of Mammals. -

A Transneuronal Analysis of the Olivocochlear and the Middle Ear Muscle Reflex Pathways
$WUDQVQHXURQDODQDO\VLVRIWKH ROLYRFRFKOHDUDQGWKHPLGGOHHDU PXVFOHUHIOH[SDWKZD\V 6XGHHS0XNHUML Dissertation for the degree of philosophiae doctor (PhD) at the University of Bergen Dissertation date: “There is nothing noble in being superior to your fellow man; true nobility is being superior to your former self.” -Ernest Hemmingway CONTENTS ________________________________________________________________________ 1. Acknowledgements……………………………………………………………..............4 2. Scientific Environment………………………………………………………................6 3. Abstract…………………………………………………………………………………7 4. List of publications……………………………………………………………............10 5. Abbreviations………………………………………………………………….............11 6. Introduction 6.1 Middle ear muscles………………………………………………............13 6.2 Middle ear muscle function……………………………………...............15 6.3 Middle ear muscle reflex…………………………………………...........17 6.4 The descending limb: motoneurons……………………………………...20 6.5 Synapses………………………………………………………………….24 6.6 Clinical applications………………………………...................................28 6.7 Clinical syndromes……………………………………………………….30 6.7 Olivocochlear reflex pathway……………………………………............32 6.8 Reflex interneurons………………………………………………............34 6.9 Transneuronal labeling of reflex pathways………………………............36 7. Study aims…………………………………………………………………….............43 8. Methodology…………………………………………………………….....................45 9. Summary of results 9.1 Study 1. Nature of labeled components of the tensor tympani muscle reflex pathway and possible non-auditory neuronal inputs……………………...49 -

Computed Tomography of the Buccomasseteric Region: 1
605 Computed Tomography of the Buccomasseteric Region: 1. Anatomy Ira F. Braun 1 The differential diagnosis to consider in a patient presenting with a buccomasseteric James C. Hoffman, Jr. 1 region mass is rather lengthy. Precise preoperative localization of the mass and a determination of its extent and, it is hoped, histology will provide a most useful guide to the head and neck surgeon operating in this anatomically complex region. Part 1 of this article describes the computed tomographic anatomy of this region, while part 2 discusses pathologic changes. The clinical value of computed tomography as an imaging method for this region is emphasized. The differential diagnosis to consider in a patient with a mass in the buccomas seteric region, which may either be developmental, inflammatory, or neoplastic, comprises a rather lengthy list. The anatomic complexity of this region, defined arbitrarily by the soft tissue and bony structures including and surrounding the masseter muscle, excluding the parotid gland, makes the accurate anatomic diagnosis of masses in this region imperative if severe functional and cosmetic defects or even death are to be avoided during treatment. An initial crucial clinical pathoanatomic distinction is to classify the mass as extra- or intraparotid. Batsakis [1] recommends that every mass localized to the cheek region be considered a parotid tumor until proven otherwise. Precise clinical localization, however, is often exceedingly difficult. Obviously, further diagnosis and subsequent therapy is greatly facilitated once this differentiation is made. Computed tomography (CT), with its superior spatial and contrast resolution, has been shown to be an effective imaging method for the evaluation of disorders of the head and neck. -

The Muscles of the Jaw Are Some of the Strongest in the Human Body
MUSCLES OF MASTICATION The muscles of the jaw are some of the strongest in the human body. They aid in chewing and speech by allowing us to open and close our mouths. Ready to unlock the mysteries of mastication? Then read on! OF MASSETERS AND MANDIBLES The deep and superficial masseter muscles enable mastication (chewing by pulling the mandible (jawbone) up towards the maxillae. Factoid! Humans’ jaws are able to bite with DEEP a force of about 150-200 psi (890 MASSETER Newtons). In contrast, a saltwater crocodile can bite with a force of 3,700 SUPERFICIAL psi (16, 400 Newtons)! MASSETER MAXILLA (R) 2 MANDIBLE MORE MASSETER FACTS The deep masseter’s origin is the zygomatic arch and the superficial masseter’s origin is the zygomatic bone. Both masseters insert into the ramus of the mandible, though the deep masseter’s insertion point is closer to the temporomandibular joint. The mandible is the only bone in the skull that we can consciously move (with the help of muscles, of course). 3 TEMPORALIS The temporalis muscles sit on either side of the head. Their job is to elevate and retract the mandible against the maxillae. They originate at the temporal fossa and temporal fascia and insert at the coronoid process and ramus of the mandible. 4 LATERAL SUPERIOR PTERYGOIDS HEAD The lateral pterygoids draw the mandibular condyle and articular disc of the temporomandibular joint forward. Each lateral pterygoid has two heads. The superior head originates at the sphenoid and infratemporal crest and the inferior head originates at the lateral pterygoid plate. -

Muscles of Mastication Muscles That Move the Head
1 Muscles Of Mastication identification origin insertion action maxilla, zygomatic arch Mandible elevates & protracts mandible MASSETER Human Cat Zygomatic Bone Mandible elevates mandible TEMPORALIS Human/Cat Temporal Bone Mandible elevates and retracts mandible Hyoid Bone DIGASTRIC Human mandible & mastoid process depress mandible Cat occipital bone & mastoid process Mandible depress mandible raises floor of mouth; MYLOHYOID Human/Cat Mandible Hyoid bone pulls hyoid forward Muscles That Move The Head identification origin insertion action STERNOCLEIDOMAStoID clavicle, sternum mastoid process flexes and laterally rotates head HUMAN ONLY STERNOMAStoID CAT ONLY sternum mastoid process turns and depresses head pulls head laterally; CLEIDOMAStoID CAT ONLY clavicle mastoid process pulls clavicle craniad 2 Muscles Of The Hyoid, Larynx And Tongue identification origin insertion action Human Sternum Hyoid depresses hyoid bone STERNOHYOID Cat costal cartilage 1st rib Hyoid pulls hyoid caudally; raises ribs and sternum sternum Throid cartilage of larynx Human depresses thyroid cartilage STERNothYROID Cat costal cartilage 1st rib Throid cartilage of larynx pulls larynx caudad elevates thyroid cartilage and Human thyroid cartilage of larynx Hyoid THYROHYOID depresses hyoid bone Cat thyroid cartilage of larynx Hyoid raises larynx GENIOHYOID Human/Cat Mandible Hyoid pulls hyoid craniad 3 Muscles That Attach Pectoral Appendages To Vertebral Column identification origin insertion action Human Occipital bone; Thoracic and Cervical raises clavicle; adducts, -

Understanding the Perioral Anatomy
2.0 ANCC CE Contact Hours Understanding the Perioral Anatomy Tracey A. Hotta , RN, BScN, CPSN, CANS gently infl ate and cause lip eversion. Injection into Rejuvenation of the perioral region can be very challenging the lateral upper lip border should be done to avoid because of the many factors that affect the appearance the fade-away lip. The client may also require injec- of this area, such as repeated muscle movement caus- tions into the vermillion border to further highlight ing radial lip lines, loss of the maxillary and mandibular or defi ne the lip. The injections may be performed bony support, and decrease and descent of the adipose by linear threading (needle or cannula) or serial tissue causing the formation of “jowls.” Environmental puncture, depending on the preferred technique of issues must also be addressed, such as smoking, sun the provider. damage, and poor dental health. When assessing a client Group 2—Atrophic lips ( Figure 2 ): These clients have for perioral rejuvenation, it is critical that the provider un- atrophic lips, which may be due to aging or genetics, derstands the perioral anatomy so that high-risk areas may and are seeking augmentation to make them look be identifi ed and precautions are taken to prevent serious more youthful. After an assessment and counseling adverse events from occurring. as to the limitations that may be achieved, a treat- ment plan is established. The treatment would begin he lips function to provide the ability to eat, speak, with injection into the wet–dry junction to achieve and express emotion and, as a sensory organ, to desired volume; additional injections may be per- T symbolize sensuality and sexuality.