Effector Mechanisms of T Cell–Mediated Immunity - Clinicalkey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Influence of Infection and Inflammation on Biomarkers of Nutritional Status
A2.4 INFLUENCE OF INFECTION AND INFLAMMATION ON BIOMARKERS OF NUTRITIONAL STATUS A2.4 Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron David I. Thurnham1 and George P. McCabe2 1 Northern Ireland Centre for Food and Health, University of Ulster, Coleraine, United Kingdom of Great Britain and Northern Ireland 2 Statistics Department, Purdue University, West Lafayette, Indiana, United States of America Corresponding author: David I. Thurnham; [email protected] Suggested citation: Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: World Health Organization. Report: Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17 September 2010. Geneva, World Health Organization, 2012. Abstract n Many plasma nutrients are influenced by infection or tissue damage. These effects may be passive and the result of changes in blood volume and capillary permeability. They may also be the direct effect of metabolic alterations that depress or increase the concentration of a nutrient or metabolite in the plasma. Where the nutrient or metabolite is a nutritional biomarker as in the case of plasma retinol, a depression in retinol concentrations will result in an overestimate of vitamin A deficiency. In contrast, where the biomarker is increased due to infection as in the case of plasma ferritin concentrations, inflammation will result in an underestimate of iron deficiency. Infection and tissue damage can be recognized by their clinical effects on the body but, unfortunately, subclinical infection or inflammation can only be recognized by measur- ing inflammation biomarkers in the blood. -

The Gut Microbiota and Inflammation
International Journal of Environmental Research and Public Health Review The Gut Microbiota and Inflammation: An Overview 1, 2 1, 1, , Zahraa Al Bander *, Marloes Dekker Nitert , Aya Mousa y and Negar Naderpoor * y 1 Monash Centre for Health Research and Implementation, School of Public Health and Preventive Medicine, Monash University, Melbourne 3168, Australia; [email protected] 2 School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane 4072, Australia; [email protected] * Correspondence: [email protected] (Z.A.B.); [email protected] (N.N.); Tel.: +61-38-572-2896 (N.N.) These authors contributed equally to this work. y Received: 10 September 2020; Accepted: 15 October 2020; Published: 19 October 2020 Abstract: The gut microbiota encompasses a diverse community of bacteria that carry out various functions influencing the overall health of the host. These comprise nutrient metabolism, immune system regulation and natural defence against infection. The presence of certain bacteria is associated with inflammatory molecules that may bring about inflammation in various body tissues. Inflammation underlies many chronic multisystem conditions including obesity, atherosclerosis, type 2 diabetes mellitus and inflammatory bowel disease. Inflammation may be triggered by structural components of the bacteria which can result in a cascade of inflammatory pathways involving interleukins and other cytokines. Similarly, by-products of metabolic processes in bacteria, including some short-chain fatty acids, can play a role in inhibiting inflammatory processes. In this review, we aimed to provide an overview of the relationship between the gut microbiota and inflammatory molecules and to highlight relevant knowledge gaps in this field. -

Eosinophil Extracellular Traps and Inflammatory Pathologies—Untangling the Web!
REVIEW published: 26 November 2018 doi: 10.3389/fimmu.2018.02763 Eosinophil Extracellular Traps and Inflammatory Pathologies—Untangling the Web! Manali Mukherjee 1*, Paige Lacy 2 and Shigeharu Ueki 3 1 Department of Medicine, McMaster University and St Joseph’s Healthcare, Hamilton, ON, Canada, 2 Department of Medicine, Alberta Respiratory Centre, University of Alberta, Edmonton, AB, Canada, 3 Department of General Internal Medicine and Clinical Laboratory Medicine, Akita University Graduate School of Medicine, Akita, Japan Eosinophils are an enigmatic white blood cell, whose immune functions are still under intense investigation. Classically, the eosinophil was considered to fulfill a protective role against parasitic infections, primarily large multicellular helminths. Although eosinophils are predominantly associated with parasite infections, evidence of a role for eosinophils in mediating immunity against bacterial, viral, and fungal infections has been recently reported. Among the mechanisms by which eosinophils are proposed to exert their protective effects is the production of DNA-based extracellular traps (ETs). Remarkably, Edited by: DNA serves a role that extends beyond its biochemical function in encoding RNA and Moncef Zouali, protein sequences; it is also a highly effective substance for entrapment of bacteria Institut National de la Santé et de la and other extracellular pathogens, and serves as valuable scaffolding for antimicrobial Recherche Médicale (INSERM), France mediators such as granule proteins from immune cells. Extracellular -

Hodgkin Lymphoma
Hodgkin Lymphoma Erica, Hodgkin lymphoma survivor Revised 2016 Publication Update Hodgkin Lymphoma The Leukemia & Lymphoma Society wants you to have the most up-to-date information about blood cancer treatment. See below for important new information that was not available at the time this publication was printed. In May 2017, the Food and Drug Administration (FDA) approved nivolumab (Opdivo®) for the treatment of adult patients with classical Hodgkin lymphoma (HL) that has relapsed or progressed after 3 or more lines of systemic therapy that includes autologous hematopoietic stem cell transplantation (HSCT). It is also approved for the treatment of adult patients with classical HL that has relapsed or progressed after autologous HSCT and brentuximab vedotin. These indications are approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. In March 2017, the Food and Drug Administration (FDA) approved pembrolizumab (Keytruda®) for the treatment of adult and pediatric patients with refractory classical Hodgkin lymphoma (cHL), or who have relapsed after 3 or more prior lines of therapy. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials. For more information, contact an Information Specialist at (800) 955-4572 or [email protected]. Information Specialists: 800.955.4572 I www.LLS.org PS57 A Message from Louis J. DeGennaro, PhD President and CEO of The Leukemia & Lymphoma Society The Leukemia & Lymphoma Society (LLS) is the world’s largest voluntary health organization dedicated to finding cures for blood cancer patients. -

Key Stage 4 Control of Immunity: Cascades of Shape Student Worksheet
Key Stage 4 The Importance of Shape Control of Immunity: Cascades of The action of enzymes is often compared to that of a lock and a key. Use your understanding of enzyme action, and the key Shape words below, to explain how enzymes work. Complimentary Specific Active site Student worksheet Enzyme-substrate-complex Catalyses Introduction Reused Activation energy Substrate Shape …………………………………………………………………………………………………… The immune system includes a powerful and intricate network of cells with the capacity to target and destroy disease causing …………………………………………………………………………………………………… microorganisms (pathogens). …………………………………………………………………………………………………… For the immune system to function effectively it must be carefully coordinated; the system must be able to identify …………………………………………………………………………………………………… pathogens and neutralise them. In each case, immune cells rely …………………………………………………………………………………………………… on the interaction of molecules with specific shapes to bring about their effect. In the following activities you will examine …………………………………………………………………………………………………… the importance of shape in the functioning of the immune …………………………………………………………………………………………………… system. …………………………………………………………………………………………………… Draw a cartoon diagram to support your answer above. www.oxfordsparks.ox.ac.uk/content/our-immune-system-battle-within Introduction to the immune system Cascades of shapes – a story of the immune system The immune system is comprised of many different cells that all Instructions work together to perform the complex task of recognising Read the information about each activity of the immune pathogens as ‘non-self’ and coordinating an appropriate system. Use this information to draw a cartoon of what is response to destroy them. going on. While there are many different cells involved, at GCSE level you Remember that the shape of molecules will be important are introduced to the two major categories of immune white and at points these will be required to fit together. -

Auto-Immune Disorders Treated with Therapeutic Apheresis
Immunology Research and Therapy Journal Open Access Research Article Auto-Immune Disorders treated with Therapeutic Apheresis Rolf Bambauer¹*, Reinhard Latza², Daniel Burgard³ and Ralf Schiel4 1Formerly: Institute for Blood Purification, Germany 2Laboratorium of Medicine, Germany 3Heart Center Duisburg, Germany 4Inselklinik Heringsdorf GmbH, Germany A R T I C L E I N F O A B S T R A C T Article history: Received: 12 June 2017 Auto-immune diseases based on an immune pathogenesis produce auto Accepted: 03 August 2017 Published: 14 August 2017 antibodies and circulating immune complexes, which cause inflammation in the Keywords: tissues of various organs. In most cases, these diseases have a bad prognosis Auto-immune disorders; Therapeutic apheresis; without treatment. Therapeutic plasma exchange with hollow fiber modules is Renal, Neurologic; Hematologic; used since more than 40 years and has led in combination with Dermatologic diseases immunosuppressive therapies to a steady increase in survival rates over the Copyright: © 2017 Bambauer R et al., last decades. Here we provide an overview of the most important pathogenic Immunol Res Ther J This is an open access article distributed aspects indicating that therapeutic apheresis can be a supportive therapy in under the Creative Commons Attribution License, which permits unrestricted use, auto immune diseases, such as renal, neurologic, hematologic and distribution, and reproduction in any medium, provided the original work is dermatologic diseases. properly cited. Introduction Citation this article: Bambauer R, Latza R, Burgard D, Schiel R. Auto-Immune Disorders The term auto immune disease relates to diseases caused by antibodies acting treated with Therapeutic Apheresis. against the body´s own tissue. -

Innate Immunity and Inflammation
ISBTc ‐ Primer on Tumor Immunology and Biological Therapy of Cancer InnateInnate ImmunityImmunity andand InflammationInflammation WillemWillem Overwijk,Overwijk, Ph.D.Ph.D. MDMD AndersonAnderson CancerCancer CenterCenter CenterCenter forfor CancerCancer ImmunologyImmunology ResearchResearch Houston,Houston, TXTX www.allthingsbeautiful.com InnateInnate ImmunityImmunity andand InflammationInflammation • Definitions • Cells and Molecules • Innate Immunity and Inflammation in Cancer • Bad Inflammation • Good Inflammation • Therapeutic Implications InnateInnate ImmunityImmunity andand InflammationInflammation • Definitions • Cells and Molecules • Innate Immunity and Inflammation in Cancer • Bad Inflammation • Good Inflammation • Therapeutic Implications • Innate Immunity: Immunity that is naturally present and is not due to prior sensitization to an antigen; generally nonspecific. It is in contrast to acquired/adaptive immunity. Adapted from Merriam‐Webster Medical Dictionary • Innate Immunity: Immunity that is naturally present and is not due to prior sensitization to an antigen; generally nonspecific. It is in contrast to acquired/adaptive immunity. • Inflammation: a local response to tissue injury – Rubor (redness) – Calor (heat) – Dolor (pain) – Tumor (swelling) Adapted from Merriam‐Webster Medical Dictionary ““InnateInnate ImmunityImmunity”” andand ““InflammationInflammation”” areare vaguevague termsterms •• SpecificSpecific cellcell typestypes andand moleculesmolecules orchestrateorchestrate specificspecific typestypes ofof inflammationinflammation -

Effect of Exercise Intensity on Cell-Mediated Immunity
sports Perspective Effect of Exercise Intensity on Cell-Mediated Immunity Katsuhiko Suzuki 1,* and Harumi Hayashida 2 1 Faculty of Sport Sciences, Waseda University, 2-579-15 Mikajima, Tokorozawa 359-1192, Japan 2 Faculty of Culture and Sport Policy, Toin University of Yokohama, 1614 Kurogane-cho, Aoba-ku, Yokohama 225-8503, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-4-2947-6898 Abstract: Moderate-intensity exercise is considered to enhance immune function and to be useful for preventing acute upper respiratory infections and similar conditions. Many people practice low- intensity short-duration exercise with the expectation of a beneficial effect on immunocompetency. However, it is difficult to affirm the existence of definite evidence of such a benefit. In this article, we discuss the effects of low-intensity short-duration exercise on cell-mediated immunity, and contrast them to the effects of high-intensity and long-duration exercise. Whereas high-intensity exercise induces inflammation and reduces cell-mediated immune system function, low-intensity exercise does not appear to have a large effect on either inflammation or cell-mediated immune function. Low-intensity exercises such as walking and yoga, which are helpful to relieve stress, cannot be considered as harmful to the immune system. Although yoga was shown to impose fewer restrictions on breathing and physical strain, the evidence that yoga enhances cell-mediated immunity remains insufficient. Therefore, further studies are needed to examine the exercise mode that may be most effective for improvement of immune functions. Keywords: exercise; walking; yoga; cellular immune system; cytokines; inflammation 1. -

An EANM Procedural Guideline
European Journal of Nuclear Medicine and Molecular Imaging https://doi.org/10.1007/s00259-018-4052-x GUIDELINES Clinical indications, image acquisition and data interpretation for white blood cells and anti-granulocyte monoclonal antibody scintigraphy: an EANM procedural guideline A. Signore1 & F. Jamar2 & O. Israel3 & J. Buscombe4 & J. Martin-Comin5 & E. Lazzeri6 Received: 27 April 2018 /Accepted: 6 May 2018 # The Author(s) 2018 Abstract Introduction Radiolabelled autologous white blood cells (WBC) scintigraphy is being standardized all over the world to ensure high quality, specificity and reproducibility. Similarly, in many European countries radiolabelled anti-granulocyte antibodies (anti-G-mAb) are used instead of WBC with high diagnostic accuracy. The EANM Inflammation & Infection Committee is deeply involved in this process of standardization as a primary goal of the group. Aim The main aim of this guideline is to support and promote good clinical practice despite the complex environment of a national health care system with its ethical, economic and legal aspects that must also be taken into consideration. Method After the standardization of the WBC labelling procedure (already published), a group of experts from the EANM Infection & Inflammation Committee developed and validated these guidelines based on published evidences. Results Here we describe image acquisition protocols, image display procedures and image analyses as well as image interpre- tation criteria for the use of radiolabelled WBC and monoclonal antigranulocyte antibodies. Clinical application for WBC and anti-G-mAb scintigraphy is also described. Conclusions These guidelines should be applied by all nuclear medicine centers in favor of a highly reproducible standardized practice. Keywords Infection . -
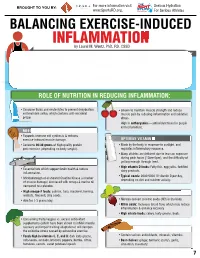
INFLAMMATION MICRONUTRIENTS SOURCES FUNCTION by Laurel M
For more information visit Serious Hydration For more information visit Serious Hydration BROUGHT TO YOU BY: BROUGHT TO YOU BY: For more information visit Serious Hydration www.SportsRD.org. For Serious Athletes www.SportsRD.org. For Serious Athletes www.SportsRD.org. NASA DEVELOPED For Serious Athletes NUTRITIONAL SUPPORT FOR INJURY BALANCING EXERCISE-INDUCED RECOVERY AND RETURN-TO-PLAY INFLAMMATION MICRONUTRIENTS SOURCES FUNCTION by Laurel M. Wentz, PhD, RD, CSSD Vitamin C Citrus fruit, red and green peppers, Antioxidant, wound healing, tissue repair, EXERCISE-INDUCED INFLAMMATION IS THE BODY’S RESPONSE TO INJURY DUE TO INTENSE PHYSICAL ACTIVITY cantaloupe immune function • The immune system response causes redness, swelling, pain. Vitamin A Sweet potato, spinach, carrots, Cell growth and development, • Acute inflammation is a normal response to high-intensity exercise, but prolonged (chronic) tomatoes immune function inflammation is a sustained response that affects the entire body. Vitamin D Sun exposure, oily fish, Promotes calcium absorption and bone health • Prolonged Inflammation: dairy products, fortified foods 1. Causes fatigue, muscle damage and soreness. 2. Limits muscle growth and training progression and increases muscle loss. Calcium Low-fat milk, fortified non-dairy milk, Supports skeletal structure and function 3. Modulating prolonged inflammation may enhance recovery & reduce soreness. low-fat Greek yogurt, cheese, broccoli, kale, fortified orange juice Magnesium Almonds, sesame and sunflower Nucleic acid and protein -

A Role of Inflammation and Immunity in Essential Hypertension—Modeled and Analyzed Using Petri Nets
International Journal of Molecular Sciences Article A Role of Inflammation and Immunity in Essential Hypertension—Modeled and Analyzed Using Petri Nets Dorota Formanowicz 1 , Agnieszka Rybarczyk 2,3 , Marcin Radom 2,3 and Piotr Formanowicz 2,3,* 1 Department of Clinical Biochemistry and Laboratory Medicine, Poznan University of Medical Sciences, 60-806 Poznan, Poland; [email protected] 2 Institute of Computing Science, Poznan University of Technology, 60-965 Poznan, Poland; [email protected] (A.R.); [email protected] (M.R.) 3 Institute of Bioorganic Chemistry, Polish Academy of Sciences, 61-704 Poznan, Poland * Correspondence: [email protected] Received: 15 April 2020; Accepted: 5 May 2020; Published: 9 May 2020 Abstract: Recent studies have shown that the innate and adaptive immune system, together with low-grade inflammation, may play an important role in essential hypertension. In this work, to verify the importance of selected factors for the development of essential hypertension, we created a Petri net-based model and analyzed it. The analysis was based mainly on t-invariants, knockouts of selected fragments of the net and its simulations. The blockade of the renin-angiotensin (RAA) system revealed that the most significant effect on the emergence of essential hypertension has RAA activation. This blockade affects: (1) the formation of angiotensin II, (2) inflammatory process (by influencing C-reactive protein (CRP)), (3) the initiation of blood coagulation, (4) bradykinin generation via the kallikrein-kinin system, (5) activation of lymphocytes in hypertension, (6) the participation of TNF alpha in the activation of the acute phase response, and (7) activation of NADPH oxidase—a key enzyme of oxidative stress. -

Products & Technology Wound Inflammation and the Role of A
Products & technology Wound inflammation and the role of a multifunctional polymeric dressing Temporary inflammation is a normal response in acute wound healing. However, in chronic wounds, the inflammatory phase is dysfunctional in nature. This results in delayed healing, and causes further problems such as increased pain, odour and Intro high levels of exudate production. It is important to choose a dressing that addresses all of these factors while meeting the patient’s needs. Multifunctional polymeric Authors: Keith F Cutting membrane dressings (e.g. PolyMem®, Ferris) can help to simplify this choice and Authors: Peter Vowden assist healthcare professionals in chronic wound care. The unique actions of xxxxx Cornelia Wiegand PolyMem® have been proven to reduce and prevent inflammation, swelling, bruising and pain to promote rapid healing, working in the deep tissues beneath the skin[1,2]. he mechanism of acute wound healing — the vascular and cellular stages. During is a well-described complex cellular vascular response, immediately on injury there is T interaction[3] that can be divided into an initial transient vasoconstriction that can be several integrated processes: haemostasis, measured in seconds. This is promptly followed inflammation, proliferation, epithelialisation by vasodilation under the influence of histamine and tissue remodeling. Inflammation is a key and nitric oxide (NO) that cause an inflow of blood. component of acute wound healing, clearing An increase in vascular permeability promotes damaged extracellular matrix, cells and debris leakage of serous fluid (protein-rich exudate) into from zones of tissue damage. This is normally a the extravascular compartment, which in turn time-limited orchestrated process. Successful increases the concentration of cells and clotting progression of the inflammatory phase allows factors.