Tobacco Harm Reduction Submission
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Spatial Distribution of Tobacco Pipe Fragments at the Hudson's Bay Company Fort Vancouver Village Site: Smoking As a Shared and Social Practice
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses Spring 6-20-2013 The Spatial Distribution of Tobacco Pipe Fragments at the Hudson's Bay Company Fort Vancouver Village Site: Smoking as a Shared and Social Practice Katie Ann Wynia Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Archaeological Anthropology Commons, and the Social and Cultural Anthropology Commons Let us know how access to this document benefits ou.y Recommended Citation Wynia, Katie Ann, "The Spatial Distribution of Tobacco Pipe Fragments at the Hudson's Bay Company Fort Vancouver Village Site: Smoking as a Shared and Social Practice" (2013). Dissertations and Theses. Paper 1085. https://doi.org/10.15760/etd.1085 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. The Spatial Distribution of Tobacco Pipe Fragments at the Hudson’s Bay Company Fort Vancouver Village Site: Smoking as a Shared and Social Practice by Katie Ann Wynia A thesis submitted in partial fulfillment of the requirements for the degree of Master of Arts in Anthropology Thesis Committee: Kenneth M. Ames, Chair Douglas C. Wilson Shelby Anderson Portland State University 2013 Abstract This thesis represents one of the first systematic, detailed spatial analyses of artifacts at the mid-19th century Hudson’s Bay Company’s Fort Vancouver Village site, and of clay tobacco pipe fragments in general. -
Talking Tobacco Harm Reduction
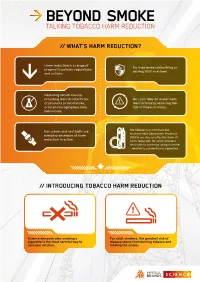
TALKING TOBACCO HARM REDUCTION // WHAT’S HARM REDUCTION? Harm reduction is a range of Do they make sunbathing or pragmatic policies, regulations driving 100% risk-free? and actions. Reducing health risks by providing less harmful forms No – but they do make them of products or substances, less harmful by reducing the or by encouraging less risky risk of illness or injury. behaviours. Sun cream and seat belts are We believe non-combustible nicotine Next Generation Products everyday examples of harm (NGPs) are also an effective form of reduction in action. harm reduction for adult smokers who wish to continue using nicotine – relative to conventional cigarettes. // INTRODUCING TOBACCO HARM REDUCTION Science demonstrates smoking a For adult smokers, the greatest risk of cigarette is the most harmful way to disease stems from burning tobacco and consume nicotine. inhaling the smoke. // WHAT IS THR? Tobacco smoke contains over 7000 The undisputed best action adult chemicals – nicotine is one of them. smokers can take to improve their Around 100 are classified by public health is to stop all tobacco and health experts as causes or potential nicotine use entirely, but many are not causes of smoking-related disease. interested or willing to take this step. Numerous public health bodies1 While the science suggests nicotine is believe transitioning to nicotine addictive and not risk-free, it’s neither products that are substantially less carcinogenic nor the primary cause of harmful than inhaled tobacco smoke smoking-related diseases. is their next best option – we agree. Contains 7000+ Contains chemicals, 100 significantly of them harmful fewer and lower or potentially levels of harmful harmful. -

Comments to the FDA on Swedish Match
November 25, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA-2014-N-1051, Modified Risk Tobacco Product Applications: Applications for 10 Products Submitted by Swedish Match North America, Inc. The undersigned organizations submit these comments in response to the announcement for public comment of a modified risk tobacco product application by Swedish Match North America, Inc. (“Swedish Match” or “the company”) for 10 tobacco products.1 The Swedish Match application presents evidence to FDA that support the proposition that Swedish smokers who switch entirely to snus derive individual health benefits. Swedish Match also provides evidence indicating that the high prevalence of snus usage among men in Sweden has contributed to a lower frequency of tobacco-related disease and mortality than is found in comparable populations with higher cigarette smoking rates. This information suggests that if Swedish Match presents convincing evidence that being permitted to make a modified risk claim would lead smokers that would not otherwise quit smoking to switch completely to one of these Swedish snus products, without leading to significant use among youth, such an application would deserve serious consideration. However, as discussed at length in Part I below, the modified risk application submitted by Swedish Match in its current form must be denied by FDA because it is legally defective under federal law. Although Swedish Match seeks a modified risk order under Section 911 of the Food, Drug & Cosmetic Act (FDCA), as amended by the Family Smoking Prevention and Tobacco Control Act (“Tobacco Control Act” or “TCA”), Swedish Match does not propose to make a claim concerning the relative risk for the listed snus products. -

An Endgame for Tobacco? by the Various Proposals
Editorial Tob Control: first published as 10.1136/tobaccocontrol-2013-050989 on 15 April 2013. Downloaded from then to evaluate what might be achieved An endgame for tobacco? by the various proposals. The latter requires contemplation of each strategy’s Kenneth E Warner potential outcomes, good and undesirable, and the political, legal, ethical, economic, regulatory and social barriers to realising ABSTRACT great successes of many national anti- the strategy’s implementation. Finally, the Since its origins in the 1960s, tobacco control smoking campaigns notwithstanding, the very idea of what constitutes the end of has achieved remarkable success against the pandemic persists. In many developed the endgame needs to be addressed. scourge of tobacco-produced disease and countries with the most robust tobacco Strikingly, to date, there has never been a death. Yet tobacco use, especially cigarette control efforts, the decline in smoking serious discussion within the tobacco smoking, remains the world's leading cause of prevalence has slowed to a trickle. control community about what would preventable premature death and is likely to Evidence-based policies seem incapable of constitute a final victory in tobacco do so for decades to come. Evidence-based substantially hastening the demise of control. Would it be a reduction in 5 policies seem incapable of substantially smoking in these countries. In LMICs, smoking-produced deaths to, say, 10% of hastening the demise of smoking. Slowness in smoking is on the rise. The net effect is current levels? -

Plain Packaging of Tobacco Products
Plain packaging of tobacco products EVIDENCE, DESIGN AND IMPLEMENTATION Plain packaging of tobacco products EVIDENCE, DESIGN AND IMPLEMENTATION Contents Executive summary vii WHO Library Cataloguing-in-Publication Data Introduction 1 Plain packaging of tobacco products: evidence, design and implementation. Part 1. Plain packaging: definition, purposes and evidence 3 1.1 A working definition of plain packaging 4 1.Tobacco Products. 2.Product Packing. 3.Tobacco Industry – legislation. Purposes of plain packaging 8 4.Health Policy. 5.Smoking – prevention and control. 6.Tobacco Use – 1.2 prevention and control. I.World Health Organization. 1.3 The evidence base underlying plain packaging 10 1.3.1 The attractiveness of tobacco products and the advertising function of branding 11 ISBN 978 92 4 156522 6 (NLM classification: WM 290) 1.3.2 Misleading tobacco packaging 12 1.3.3 The effectiveness of health warnings 13 1.3.4 The prevalence of tobacco use 13 © World Health Organization 2016 1.3.5 Expert reviews of the evidence 15 1.3.6 Conclusions 18 All rights reserved. Publications of the World Health Organization are Additional resources 19 available on the WHO website (http://www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; Part 2. Policy design and implementation 21 email: [email protected]). 2.1 The policy design process 22 2.2 Implementation of plain packaging 25 Requests for permission to reproduce or translate WHO publications 2.3 Compliance and enforcement 32 –whether for sale or for non-commercial distribution– should be 2.3.1 Delayed compliance and penalties for non-compliance 33 addressed to WHO Press through the WHO website (http://www.who.int/ 2.3.2 Sleeves, stickers, inserts and other devices 34 about/licensing/copyright_form/index.html). -

A FOCUS on HARM REDUCTION Why It
A FOCUS ON HARM REDUCTION Why it mattersSUSTAINABILITY FOCUS REPORT 2013: How we address the public health impact of our products A nicotine molecule In this report Focusing on the facts 2 At the core of our business strategy 3 Innovative nicotine products 4 ‘Safer’ tobacco products: the research 7 So, what’s next? 9 Our Chief Executive on why it matters b British American Tobacco Harm reduction: sustainability focus report 2013 Because it’s crucial to the future of our business. Surely tobacco harm reduction should just be about What areas are you concentrating on? getting people to quit smoking? Our approach to harm reduction has two distinct areas: The only way to be certain of avoiding the serious health risks nicotine-based alternatives and reduced-risk tobacco products. associated with smoking is not to smoke at all. However, despite In the nicotine category, we have established a stand-alone increasingly strict tobacco control policies, many people continue business solely dedicated to this area. This brings together our to smoke. And the World Health Organisation estimates that existing Nicoventures business with CN Creative, the e-cigarette 1 many more will do so in the future . So realistically the ‘quit or die’ company we acquired at the end of last year, into a single business approach to reducing the public health impact of smoking simply which will continue to operate under the Nicoventures name. isn’t enough. This business has already launched its first e-cigarette in the UK, For adults that choose to continue to smoke, tobacco harm which will be expanded into further markets in the coming year. -

List of References Submitted During the Public Consultation
Bibliography of the Sources Examined for the Opinion on the Addictiveness and Attractiveness of Tobacco Additives ACSCAN (American Cancer Society Cancer Action Network), American Heart Association, American Lung Association, Campaign for Tobacco-Free Kids, American Legacy Foundation. Submission to U.S. Food and Drug Administration re Docket No. FDA- 2010-N-0207, 2010; July 26. Available from: URL: http://www.tobaccofreekids.org/reports/fda/regulatory_docs/Comments%20to%20 FDA%20RFI%20re%20youth%20and%20minority%20marketing%207-26-10.pdf Adam T, McAughey J, McGrath C, Mocker C, Zimmermann R. Simultaneous on-line size and chemical analysis of gas phase and particulate phase of cigarette mainstream smoke. Anal Bioanal Chem 2009; 394:1193-203. Adam T, Mitschke S, Streibel T, Baker RR, Zimmermann R. Puff-by-puff resolved characterisation of cigarette mainstream smoke by single photon ionisation (SPI)- time-of-flight mass spectrometry (TOFMS): comparison of the 2R4F research cigarette and pure Burley, Virginia, Oriental and Maryland tobacco cigarettes. Anal Chim Acta. 2006 Jul 21;572(2):219-29. Epub 2006; May 22. Ahijevych K, Garrett BE. Menthol pharmacology and its potential impact on cigarette smoking behaviour. Nicotine Tob Res 2004; 6 Suppl 1:S17-28. Ahijevych K, Gillespie J, Demirci M, Jagadeesh J. Menthol and nonmenthol cigarettes and smoke exposure in black and white women. Pharmacol Biochem Behav 1996; 53:355-60. Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav 1999; 24:115-20. Akehurst BC Tobacco. Second edition. Tropical Agriculture Series. Longman. London and New York. 1981; Pp. -

Tobacco Control
TOBACCO CONTROL Playbook World Health Organization ABSTRACT Tobacco control is difficult and complex and obstructed by the tactics of the tobacco industry and its allies to oppose effective tobacco control measures. This document was developed by the WHO Regional Office for Europe by collecting numerous evidence-based arguments from different thematic areas, reflecting the challenges that tobacco control leaders have faced while implementing various articles of the WHO FCTC and highlighting arguments they have developed in order to counter and succeed against the tobacco industry. KEY WORDS TOBACCO CONTROL WHO FCTC HEALTH EFFECTS TOBACCO INDUSTRY ARGUMENTS © World Health Organization 2019 All rights reserved. The Regional Office for Europe of the World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. -

Talking About the Smokes MJA Transforming the Evidence to Guide Aboriginal
Talking About The Smokes MJA Transforming the evidence to guide Aboriginal 1 JUNE 2015 VOLUME 202 NO 10 and Torres Strait Islander tobacco control T H E M E D I C A L J O U R N A L O F A U S T R A L I A SUPPLEMENT Journal of the Australian Medical Association • Established in 1914 Print Post Approved PP255003/00505 Supplement 010615.indb 1 22/05/2015 7:44:21 AM Talking About The Smokes (TATS) is a model for how to do a large national epidemiological project in partnership with Aboriginal communities, the National Aboriginal Community Controlled Health Organisation (NACCHO) and the Aboriginal community-controlled health service (ACCHS) sector. Research has not always been done well in or in partnership with Aboriginal and Torres Strait Islander communities, which can make undertaking research with the sector challenging. The TATS project, however, has always felt like a full and respectful partnership between the ACCHS sector and research organisations, and between Aboriginal and non- Aboriginal people. We have appreciated our involvement in all elements of the project, the clarity of the formal agreements, and the funding and support of project staff employed at NACCHO and in our member ACCHSs. Our concerns and priorities were always addressed. The ACCHS sector recognises how important undertaking research is to reduce smoking in our communities. Because TATS has been done ethically, we can have confi dence in using the evidence from this project to improve our policies and programs to reduce the damage that smoking does to our people and communities. -

The Health Effects and Regulation of Passive Smoking a Submission to the NH&MRC Health Care Committee
OFFICE OF REGULATION REVIEW INDUSTRY COMMISSION The health effects and regulation of passive smoking A submission to the NH&MRC Health Care Committee SUB MIS SIO N APR IL 199 4 INTRODUCTION The Office of Regulation Review (ORR) — located within the Industry Commission — is responsible for administering the Commonwealth Government’s regulation review program. Amongst other functions, the ORR is required to ensure that proposals for new or amended business regulation meet with the Government’s policy on regulation. Details of the ORR and the regulation review procedures are attached. The Health Care Committee of the National Health and Medical Research Council (NH&MRC) has published a Notice of Intent to issue guidelines or make regulatory recommendations regarding passive smoking, and has invited submissions on this issue. The regulation to be considered by Committee includes forms of business regulation, and thus falls within the scope of the regulation review policy. Some issues concerning the regulation of passive smoking were discussed by the Industry Commission (1994) in its recent Draft Report on The Tobacco Growing and Manufacturing Industries. The ORR had input into that document. In this submission, the ORR seeks to assist the Committee by: · setting out aspects of the regulation review policy relevant to the Committee’s deliberations; · highlighting aspects of the Industry Commission’s Draft Report relevant to the passive smoking issue; and · expanding on those points in some cases. HEALTH EFFECTS OF PASSIVE SMOKING The first two points in the Committee’s terms of reference require it to: · review the relevant scientific evidence linking passive smoking to disease in adults and children; and · estimate the extent and impact of any illness found likely to be due to passive smoking in Australia. -

Tobacco Harm Reduction
Tobacco Harm Reduction Brad Rodu Professor, Department of Medicine James Graham Brown Cancer Center University of Louisville The Smoking Status Quo: Unacceptable • The American Anti-Smoking Campaign is 45 Years Old • According to the CDC: 45 million smokers in the U.S. 443,000 deaths every year in the U.S. 5,800 in Oklahoma Lung Cancer (ICD 161-162) Mortality in Men and Women Age 35+, Oklahoma and the US, 1979-2009 250 OK Men 200 150 US Men OK Women 100 Deaths per 100,000 py 100,000 Deathsper US Women 50 0 Year If the Status Quo Continues In the next 20 years: • 8 million Americans will die from smoking All are adults over 35 years of age None of them are now children The Failed Anti-Smoking Campaign • The Campaign’s Only Message: Quit Nicotine and Tobacco, or Die • The Campaign’s Only Quitting Tactics: Ineffective Behavioral Therapy Ineffective Use of Nicotine Rodu and Cole. Technology 6: 17-21, 1999. Rodu and Cole. International J Cancer 97: 804-806, 2002. The Anti-Smoking Campaign- Behavioral Therapy • NCI Manual for Physicians- Counsel Patients to: – ”Keep your hands busy- doodle, knit, type a letter” – ”Cut a drinking straw into cigarette-sized pieces and inhale air” – ”Keep a daydream ready to go” Source: How to help your patients stop smoking. NIH Pub. No. 93-3064, 1993 The Anti-Smoking Campaign- Faulted Use of Nicotine • Temporary – 6 to 12 weeks • Expensive – per unit and per box • Very Low Dose – unsatisfying for smokers • 7% Success* – ”Efficacious”, ”Modest” *Hughes et al. Meta-analysis in Tobacco Control, 2003. -

Cigarette Smoking, Social Support, and Workplace Smoke-Free Policies Among an Urban American Indian Population
Cigarette Smoking, Social Support, and Workplace Smoke-free Policies among an Urban American Indian Population A Dissertation SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Genelle Ruth Sanders Lamont IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Patricia M. McGovern, Ph.D., MPH, BSN, Advisor Jean L. Forster, Ph.D., MPH, Co-Advisor December 2017 © Genelle Ruth Sanders Lamont 2017 Acknowledgements I want to thank my advisors Dr. Pat McGovern and Dr. Jean Forster for their overwhelming support, dedication, advice, and help throughout this project. I would also like to thank committee members Dr. Nancy Nachreiner and Dr. Jeff Mandel for their thorough review of and advice on my project proposal and dissertation. Special thanks to Rose Hilk for helping me with data management and cleaning and Amanda Corbett and Lisa Skjefte for their hard work coordinating interviewer training and survey implementation. Chi mii-gwetch (many thanks) to Kris Rhodes, John Poupart, and Melanie Peterson-Hickey for connecting me with culturally sensitive methodologies and tobacco research in the American Indian community. I also extend my utmost gratitude to Andy Ryan for helping me to understand Directed Acyclic Graphs, regression models, and for SAS analyses troubleshooting. Support for this effort was provided, in part, by the National Institute for Occupational Safety and Health (NIOSH)’s Midwest Center for Occupational Health and Safety (#T42OH008434) and the Great Lakes Inter-Tribal Council Native American Research Center for Health. The Tribal Tobacco Use Prevalence Study was supported by ClearWay MinnesotaSM CARA Grant (RC-2008-0014). Also special thanks to community interviewers Lucy Arias, Deanna Beaulieu, Cameron Blacksmith, Christine Damann, Carl Fransen, Miigis Gonzalez, Indi Lawrence, Carrie Owen, Joy Rivera, Loretta Rivera, Rica Rivera, Sandra Rivera, Lisa Skjefte, Lucie Skjefte, Carla Smith, Samirya Strong, Corrie Thompson, Rachel Thompson, Felicia Wesaw, and Jacque Wilson.