Capture and Fusion Beats During Atrial Fibrillation and Ventricular Tachycardia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Constrictive Pericarditis Causing Ventricular Tachycardia.Pdf
EP CASE REPORT ....................................................................................................................................................... A visually striking calcific band causing monomorphic ventricular tachycardia as a first presentation of constrictive pericarditis Kian Sabzevari 1*, Eva Sammut2, and Palash Barman1 1Bristol Heart Institute, UH Bristol NHS Trust UK, UK; and 2Bristol Heart Institute, UH Bristol NHS Trust UK & University of Bristol, UK * Corresponding author. Tel: 447794900287; fax: 441173425926. E-mail address: [email protected] Introduction Constrictive pericarditis (CP) is a rare condition caused by thickening and stiffening of the pericar- dium manifesting in dia- stolic dysfunction and enhanced interventricu- lar dependence. In the developed world, most cases are idiopathic or are associated with pre- vious cardiac surgery or irradiation. Tuberculosis remains a leading cause in developing areas.1 Most commonly, CP presents with symptoms of heart failure and chest discomfort. Atrial arrhythmias have been described as a rare pre- sentation, but arrhyth- mias of ventricular origin have not been reported. Figure 1 (A) The 12 lead electrocardiogram during sustained ventricular tachycardia is shown; (B and C) Case report Different projections of three-dimensional reconstructions of cardiac computed tomography demonstrating a A 49-year-old man with a striking band of calcification around the annulus; (D) Carto 3DVR mapping—the left hand panel (i) demonstrates a background of diabetes, sinus beat with late potentials at the point of ablation in the coronary sinus, the right hand panel (iii) shows the hypertension, and hyper- pacemap with a 89% match to the clinical tachycardia [matching the morphology seen on 12 lead ECG (A)], and cholesterolaemia and a the middle panel (ii) displays the three-dimensional voltage map. -

What Are Premature Ventricular Contractions?
What Are Premature Ventricular Contractions? Premature ventricular contractions (VPCs or PVCs) are irregular beats that originate from the heart’s pumping chambers (ventricles). These beats interrupt the normal regular (sinus) rhythm, and result in irregularity to the heart rhythm. Although single PVCs are not life- threatening, they may indicate the presence of underlying heart disease, or when they become more severe can cause rapid and dangerous increases in heart rate (ventricular tachycardia). WHAT CAUSES PREMATURE VENTRICULAR CONTRACTIONS? PVCs can occur secondary to a variety of causes, most concerning being cardiac disease. PVCs are most commonly seen in dogs with Dilated Cardiomyopathy, but can also occur in the later stages of disease with Myxomatous Mitral Valve Degeneration. PVCs and other ventricular arrhythmias can also with no evidence of underlying structural heart disease. The workup for PVCs can be frustrating, because PVCs can also occur secondary to conditions unrelated to the heart. Most commonly they can occur secondary to gastrointestinal disease, systemic disease, and pain. HOW ARE PREMATURE VENTRICULAR CONTRACTIONS DIAGNOSED? Most commonly, your veterinarian will hear an irregular heart rhythm and recommend an electrocardiogram (ECG) to assess the heart rhythm. The ECG will enable your veterinarian to determine whether the irregular rhythm is due to PVCs. Following this diagnosis, there are several additional recommended tests. TESTING Following this diagnosis, there are several additional recommended tests. ECHOCARDIOGRAPHY Echocardiography (heart ultrasound) is recommended to make sure that there is no evidence of structural cardiac disease causing the abnormal heart rhythm. 24-HOUR HOLTER Single PVCs usually do not require treatment, however a Holter monitor will determine whether the frequency or severity of the MONITOR PVCs warrant anti-arrhythmic medication, and make sure that there is no evidence of ventricular tachycardia, which is a life threatening heart rhythm. -

Basic Rhythm Recognition
Electrocardiographic Interpretation Basic Rhythm Recognition William Brady, MD Department of Emergency Medicine Cardiac Rhythms Anatomy of a Rhythm Strip A Review of the Electrical System Intrinsic Pacemakers Cells These cells have property known as “Automaticity”— means they can spontaneously depolarize. Sinus Node Primary pacemaker Fires at a rate of 60-100 bpm AV Junction Fires at a rate of 40-60 bpm Ventricular (Purkinje Fibers) Less than 40 bpm What’s Normal P Wave Atrial Depolarization PR Interval (Normal 0.12-0.20) Beginning of the P to onset of QRS QRS Ventricular Depolarization QRS Interval (Normal <0.10) Period (or length of time) it takes for the ventricles to depolarize The Key to Success… …A systematic approach! Rate Rhythm P Waves PR Interval P and QRS Correlation QRS Rate Pacemaker A rather ill patient……… Very apparent inferolateral STEMI……with less apparent complete heart block RATE . Fast vs Slow . QRS Width Narrow QRS Wide QRS Narrow QRS Wide QRS Tachycardia Tachycardia Bradycardia Bradycardia Regular Irregular Regular Irregular Sinus Brady Idioventricular A-Fib / Flutter Bradycardia w/ BBB Sinus Tach A-Fib VT PVT Junctional 2 AVB / II PSVT A-Flutter SVT aberrant A-Fib 1 AVB 3 AVB A-Flutter MAT 2 AVB / I or II PAT PAT 3 AVB ST PAC / PVC Stability Hypotension / hypoperfusion Altered mental status Chest pain – Coronary ischemic Dyspnea – Pulmonary edema Sinus Rhythm Sinus Rhythm P Wave PR Interval QRS Rate Rhythm Pacemaker Comment . Before . Constant, . Rate 60-100 . Regular . SA Node Upright in each QRS regular . Interval =/< leads I, II, . Look . Interval .12- .10 & III alike .20 Conduction Image reference: Cardionetics/ http://www.cardionetics.com/docs/healthcr/ecg/arrhy/0100_bd.htm Sinus Pause A delay of activation within the atria for a period between 1.7 and 3 seconds A palpitation is likely to be felt by the patient as the sinus beat following the pause may be a heavy beat. -

PAROXYSMAL VENTRICULAR TACHYCARDIA OCCURRING in a NORMAL HEART by DAVID ROMNEY, M.B., B.Ch.(Dub.) Ex-Senior House Officer in Medicine, St
Postgrad Med J: first published as 10.1136/pgmj.31.354.191 on 1 April 1955. Downloaded from I9I -J PAROXYSMAL VENTRICULAR TACHYCARDIA OCCURRING IN A NORMAL HEART By DAVID ROMNEY, M.B., B.Ch.(Dub.) Ex-Senior House Officer in Medicine, St. James's Hospital, Balham It is widely taught-and rightly so-that by sinus pressure will, of course, not affect the paroxysmal ventricular tachycardia is one of the rate in ventricular tachycardia. rarer arrhythmias, and is associated with grave In I953 Froment, Gallavardin and Cahen myocardial damage, with special reference to myo- offered a classification of various forms of cardial infarction. It is the least common, and paroxysmal ventricular tachycardia, and included most serious of the paroxysmal tachycardias, some case report. They described the following which contain four varieties of arrhythmia: supra- groups:- ventricular (auricular and nodal) 60 per cent.; (i) Terminal prefibrillatory ventricular tachy- auricular fibrillation, 30 per cent.; auricular flutter, cardia. 6 per cent.; and ventricular tachycardia, 4 per (ii) Curable and mild monomorphic extra- cent. systoles, with paroxysms of tachycardia. Figures from various sources (Campbell, 1947) (iii) Paroxysmal ventricular tachycardia due toby copyright. would indicate that in the latter group, four-fifths a lesion of the ventricular septum. of the cases had seriously damaged hearts. In (iv) Persistent and prolonged ventricular tachy- the remaining fifth no cause was found for the cardia developing in sound hearts, usually paroxysms. Paroxysmal ventricular tachycardia is in young subjects. more common in men than in women in the proportion of about 3.2. This arrhythmia was Case Report first identified by Sir Thomas Lewis in I909 and A married woman, aged 48, first became aware Gallavardin (1920, I921, 1922) emphasized the in 1948 of a paroxysm which caused alarm, faint- seriousness of the 'terminal ven- ness and It lasted a short pre-fibrillatory collapse. -

The Example of Short QT Syndrome Jules C
Hancox et al. Journal of Congenital Cardiology (2019) 3:3 Journal of https://doi.org/10.1186/s40949-019-0024-7 Congenital Cardiology REVIEW Open Access Learning from studying very rare cardiac conditions: the example of short QT syndrome Jules C. Hancox1,4* , Dominic G. Whittaker2,3, Henggui Zhang4 and Alan G. Stuart5,6 Abstract Background: Some congenital heart conditions are very rare. In a climate of limited resources, a viewpoint could be advanced that identifying diagnostic criteria for such conditions and, through empiricism, effective treatments should suffice and that extensive mechanistic research is unnecessary. Taking the rare but dangerous short QT syndrome (SQTS) as an example, this article makes the case for the imperative to study such rare conditions, highlighting that this yields substantial and sometimes unanticipated benefits. Genetic forms of SQTS are rare, but the condition may be under-diagnosed and carries a risk of sudden death. Genotyping of SQTS patients has led to identification of clear ion channel/transporter culprits in < 30% of cases, highlighting a role for as yet unidentified modulators of repolarization. For example, recent exome sequencing in SQTS has identified SLC4A3 as a novel modifier of ventricular repolarization. The need to distinguish “healthy” from “unhealthy” short QT intervals has led to a search for additional markers of arrhythmia risk. Some overlap may exist between SQTS, Brugada Syndrome, early repolarization and sinus bradycardia. Genotype-phenotype studies have led to identification of arrhythmia substrates and both realistic and theoretical pharmacological approaches for particular forms of SQTS. In turn this has increased understanding of underlying cardiac ion channels. -

Ventricular Tachycardia Drugs Versus Devices John Camm St
Cardiology Update 2015 Davos, Switzerland: 8-12th February 2015 Ventricular Arrhythmias Ventricular Tachycardia Drugs versus Devices John Camm St. George’s University of London, UK Imperial College, London, UK Declaration of Interests Chairman: NICE Guidelines on AF, 2006; ESC Guidelines on Atrial Fibrillation, 2010 and Update, 2012; ACC/AHA/ESC Guidelines on VAs and SCD; 2006; NICE Guidelines on ACS and NSTEMI, 2012; NICE Guidelines on heart failure, 2008; NICE Guidelines on Atrial Fibrillation, 2006; ESC VA and SCD Guidelines, 2015 Steering Committees: multiple trials including novel anticoagulants DSMBs: multiple trials including BEAUTIFUL, SHIFT, SIGNIFY, AVERROES, CASTLE- AF, STAR-AF II, INOVATE, and others Events Committees: one trial of novel oral anticoagulants and multiple trials of miscellaneous agents with CV adverse effects Editorial Role: Editor-in-Chief, EP-Europace and Clinical Cardiology; Editor, European Textbook of Cardiology, European Heart Journal, Electrophysiology of the Heart, and Evidence Based Cardiology Consultant/Advisor/Speaker: Astellas, Astra Zeneca, ChanRX, Gilead, Merck, Menarini, Otsuka, Sanofi, Servier, Xention, Bayer, Boehringer Ingelheim, Bristol- Myers Squibb, Daiichi Sankyo, Pfizer, Boston Scientific, Biotronik, Medtronic, St. Jude Medical, Actelion, GlaxoSmithKline, InfoBionic, Incarda, Johnson and Johnson, Mitsubishi, Novartis, Takeda Therapy for Ventricular Tachycardia Medical therapy Antiarrhythmic drugs Autonomic management Ventricular tachycardia Monomorphic Polymorphic Ventricular fibrillation Ventricular storms Ablation therapy Device therapy Surgical Defibrillation Catheter Antitachycardia pacing History of Antiarrhythmic Drugs 1914 - Quinidine 1950 - Lidocaine 1951 - Procainamide 1946 – Digitalis 1956 – Ajmaline 1962 - Verapamil 1962 – Disopyramide 1964 - Propranolol 1967 – Amiodarone 1965 – Bretylium 1972 – Mexiletine 1973 – Aprindine, Tocainide 1969 - Diltiazem 1975- Flecainide 1976 – Propafenone Encainide Ethmozine 2000 - Sotalol D-sotalol 1995 - Ibutilide (US) Recainam 2000 – Dofetilide US) IndecainideX Etc. -

Ventricular Tachycardia Associated Syncope in a Patient of Variant
Case Report http://dx.doi.org/10.4070/kcj.2016.46.1.102 Print ISSN 1738-5520 • On-line ISSN 1738-5555 Korean Circulation Journal Ventricular Tachycardia Associated Syncope in a Patient of Variant Angina without Chest Pain Soo Jin Kim, MD, Ji Young Juong, MD, and Tae-Ho Park, MD Department of Cardiology, Dong-A University Medical Center, Busan, Korea A 68-year-old man was admitted for a syncope workup. After routine evaluation, he was diagnosed with syncope of an unknown cause and was discharged from the hospital. He was readmitted due to dizziness. On repeated Holter monitoring, polymorphic ventricular tachycardia was detected during syncope. We performed intracoronary ergonovine provocation test; severe coronary spasm was induced at 70% stenosis of the proximal left anterior descending artery. The patient was treated with percutaneous coronary intervention. We present a rare case of syncope induced by ventricular arrhythmia in a patient with variant angina without chest pain. (Korean Circ J 2016;46(1):102-106) KEY WORDS: Prinzmetal’s variant angina; Coronary vasospasm; Tachycardia, ventricular. Introduction Case Variant angina commonly manifests as chest pain and transient A 68-year-old man visited our emergency room due to recurrent ST elevation by coronary spasm, and generally follows a benign syncope. He had experienced four episodes of syncope with clinical course.1) Rarely, syncope induced by ventricular arrhythmia dizziness and chest discomfort during the prior 2 months. The associated with transient myocardial ischemia can be developed episodes were evoked when he was preparing his boat for sailing by coronary spasm.2)3) If the cause of syncope is not correctly in early morning. -

Update on the Diagnosis and Management of Familial Long QT Syndrome
Heart, Lung and Circulation (2016) 25, 769–776 POSITION STATEMENT 1443-9506/04/$36.00 http://dx.doi.org/10.1016/j.hlc.2016.01.020 Update on the Diagnosis and Management of Familial Long QT Syndrome Kathryn E Waddell-Smith, FRACP a,b, Jonathan R Skinner, FRACP, FCSANZ, FHRS, MD a,b*, members of the CSANZ Genetics Council Writing Group aGreen Lane Paediatric and Congenital Cardiac Services, Starship Children’s Hospital, Auckland New Zealand bDepartment[5_TD$IF] of Paediatrics,[6_TD$IF] Child[7_TD$IF] and[8_TD$IF] Youth[9_TD$IF] Health,[10_TD$IF] University of Auckland, Auckland, New Zealand Received 17 December 2015; accepted 20 January 2016; online published-ahead-of-print 5 March 2016 This update was reviewed by the CSANZ Continuing Education and Recertification Committee and ratified by the CSANZ board in August 2015. Since the CSANZ 2011 guidelines, adjunctive clinical tests have proven useful in the diagnosis of LQTS and are discussed in this update. Understanding of the diagnostic and risk stratifying role of LQTS genetics is also discussed. At least 14 LQTS genes are now thought to be responsible for the disease. High-risk individuals may have multiple mutations, large gene rearrangements, C-loop mutations in KCNQ1, transmembrane mutations in KCNH2, or have certain gene modifiers present, particularly NOS1AP polymorphisms. In regards to treatment, nadolol is preferred, particularly for long QT type 2, and short acting metoprolol should not be used. Thoracoscopic left cardiac sympathectomy is valuable in those who cannot adhere to beta blocker therapy, particularly in long QT type 1. Indications for ICD therapies have been refined; and a primary indication for ICD in post-pubertal females with long QT type 2 and a very long QT interval is emerging. -

Management of Wolff-Parkinson-White
Management of Wolff-Parkinson-White Tachyarrhythmia Presenting as Syncope with Seizure- like Activity * * Samuel Kaplan, BS and Lindsey Spiegelman, MD *University of California, Irvine, Department of Emergency Medicine, Orange, CA Correspondence should be addressed to Samuel Kaplan at [email protected] Submitted: August 15, 2017; Accepted: September 13, 2017; Electronically Published: October 15, 2017; https://doi.org/10.21980/J8534P Copyright: © 2017 Kaplan, et al. This is an open access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) License. See: http://creativecommons.org/licenses/by/4.0/ Empty Line Calibri Size 12 Empty Line Calibri Size 12 ABSTRACT: Audience: Emergency medicine residents and medical students. Introduction: An estimated 3% of the United States population suffers from recurrent convulsive episodes that are most often attributed to primary epileptic seizures.1 However, recent studies have estimated about 20%-30% of such episodes are associated with occult cardiac etiology,2 which carry one-year mortality rates of up to 30%.3 Cardiogenic cerebral hypoxia has been associated with a wide variety of neurologic disturbances, including dizzy spells, headache, syncope, focal motor deficit, generalized tonic-clonic seizure, confusion, dementia, and psychosis.4 Convulsive activity has tentatively been ascribed to the ensuing activation of the medullary reticular formation.5,6 This scenario is based on a patient that presented to University of California Irvine Medical Center Emergency -

An Extremely Rare Cause of Wolff-Parkinson
108 Erciyes Med J 2019; 41(1): 108–10 • DOI: 10.14744/etd.2018.18165 An Extremely Rare Cause of Wolff-Parkinson-White Syndrome: Rhabdomyoma in Association With Tuberous Sclerosis CASE REPORT Özlem Elkıran , Cemşit Karakurt , Damla İnce ABSTRACT Rhabdomyomas are the most common primary cardiac tumors in infants and children. They are usually associated with tuberous sclerosis (TS). As the tumors tend to regress spontaneously, surgical intervention is not usually performed unless they become obstructive or cause incessant arrhythmias. We report an extremely rare case of rhabdomyoma serving as a substrate for Wolff-Parkinson-White (WPW) syndrome and intractable supraventricular tachycardia accompanied by TS. Our case is particularly interesting because it was diagnosed prenatally. The signs of WPW syndrome disappeared from the elec- trocardiogram with the regression of the tumor. Keywords: Wolff-Parkinson-White Syndrome, child, rhabdomyoma INTRODUCTION Rhabdomyomas are the most common cardiac tumors in infants and children, and they are closely related with tuberous sclerosis (TS). A significant part of rhabdomyomas is asymptomatic, and they regress on follow-up. However, symptoms of cardiac failure, arrhythmias, and obstruction can be observed depending on their location in the heart. They require urgent medical or surgical treatment (1, 2). Cite this article as: Elkıran Ö, Karakurt C, İnce D. An Extremely Rhabdomyoma-related arrhythmias are reported as premature atrial and ventricular contractions, supraventricular Rare Cause of and ventricular tachycardia, sinus node dysfunction, atrioventricular block, and Wolff-Parkinson-White (WPW) Wolff-Parkinson-White syndrome (1, 3, 4). There are only a few studies of WPW syndrome occurring in association with TS, with and Syndrome: Rhabdomyoma in Association With without rhabdomyoma. -

Cardiac Characteristics in the Premature Ventricular Contraction Patients with Or Without Ventricular Tachycardia
Int J Clin Exp Med 2018;11(6):6106-6112 www.ijcem.com /ISSN:1940-5901/IJCEM0064109 Original Article Cardiac characteristics in the premature ventricular contraction patients with or without ventricular tachycardia Yafen Su1*, Meng Xia2,3*, Junxian Cao2, Qianping Gao2 1Ambulatory Electrocardiogram Room, The First Affiliated Hospital of Harbin Medical University, China; 2Unit of Cardiology, The First Affiliated Hospital of Harbin Medical University, China; 3Unit of Cardiology, Liaoyang Central Hospital, China. *Equal contributors. Received August 21, 2017; Accepted March 22, 2018; Epub June 15, 2018; Published June 30, 2018 Abstract: Background/Aims: Frequent (sustained) premature ventricular contractions (PVCs) lead to ventricu- lar tachycardia (VT), which triggers ventricular fibrillation and sudden cardiac death. The cardiac characteristics and risk prediction in frequent/sustained PVC patients with or without VT have been still in need of investigation. Methods: The data from patients with frequent PVCs via 24 h ambulatory electrocardiogram (ECG) monitor were collected at the Department of Cardiology in the First Affiliated Hospital of Harbin Medical University from January 1, 2012 to August 31, 2015. Total 342 patients were grouped into VT group (n=136) and Non-VT group (n=206) based on the presence or absence of VT. Cardiac functional examination and blood tests were carried out on the second day of admission. Independent risk factors related to the occurrence of VT were identified. The receiver operating characteristic (ROC) curves was established to evaluate the accuracy of the risk factors and VT. Results: The baseline characteristics were similar between the two groups. The blood potassium, extensive PVC burden, left ventricular ejection fraction (LVEF), PVC couplets, and alcohol consumption were associated with the occurrence of VT. -
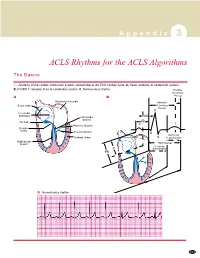
ACLS Rhythms for the ACLS Algorithms
A p p e n d i x 3 ACLS Rhythms for the ACLS Algorithms The Basics 1. Anatomy of the cardiac conduction system: relationship to the ECG cardiac cycle. A, Heart: anatomy of conduction system. B, P-QRS-T complex: lines to conduction system. C, Normal sinus rhythm. Relative Refractory A B Period Bachmann’s bundle Absolute Sinus node Refractory Period R Internodal pathways Left bundle AVN branch AV node PR T Posterior division P Bundle of His Anterior division Q Ventricular Purkinje fibers S Repolarization Right bundle branch QT Interval Ventricular P Depolarization PR C Normal sinus rhythm 253 A p p e n d i x 3 The Cardiac Arrest Rhythms 2. Ventricular Fibrillation/Pulseless Ventricular Tachycardia Pathophysiology ■ Ventricles consist of areas of normal myocardium alternating with areas of ischemic, injured, or infarcted myocardium, leading to chaotic pattern of ventricular depolarization Defining Criteria per ECG ■ Rate/QRS complex: unable to determine; no recognizable P, QRS, or T waves ■ Rhythm: indeterminate; pattern of sharp up (peak) and down (trough) deflections ■ Amplitude: measured from peak-to-trough; often used subjectively to describe VF as fine (peak-to- trough 2 to <5 mm), medium-moderate (5 to <10 mm), coarse (10 to <15 mm), very coarse (>15 mm) Clinical Manifestations ■ Pulse disappears with onset of VF ■ Collapse, unconsciousness ■ Agonal breaths ➔ apnea in <5 min ■ Onset of reversible death Common Etiologies ■ Acute coronary syndromes leading to ischemic areas of myocardium ■ Stable-to-unstable VT, untreated ■ PVCs with