Terminologia Histologica 10 Years On: Some Disputable Terms in Need
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
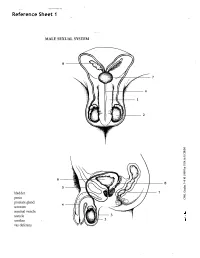
Reference Sheet 1
MALE SEXUAL SYSTEM 8 7 8 OJ 7 .£l"00\.....• ;:; ::>0\~ <Il '"~IQ)I"->. ~cru::>s ~ 6 5 bladder penis prostate gland 4 scrotum seminal vesicle testicle urethra vas deferens FEMALE SEXUAL SYSTEM 2 1 8 " \ 5 ... - ... j 4 labia \ ""\ bladderFallopian"k. "'"f"";".'''¥'&.tube\'WIT / I cervixt r r' \ \ clitorisurethrauterus 7 \ ~~ ;~f4f~ ~:iJ 3 ovaryvagina / ~ 2 / \ \\"- 9 6 adapted from F.L.A.S.H. Reproductive System Reference Sheet 3: GLOSSARY Anus – The opening in the buttocks from which bowel movements come when a person goes to the bathroom. It is part of the digestive system; it gets rid of body wastes. Buttocks – The medical word for a person’s “bottom” or “rear end.” Cervix – The opening of the uterus into the vagina. Circumcision – An operation to remove the foreskin from the penis. Cowper’s Glands – Glands on either side of the urethra that make a discharge which lines the urethra when a man gets an erection, making it less acid-like to protect the sperm. Clitoris – The part of the female genitals that’s full of nerves and becomes erect. It has a glans and a shaft like the penis, but only its glans is on the out side of the body, and it’s much smaller. Discharge – Liquid. Urine and semen are kinds of discharge, but the word is usually used to describe either the normal wetness of the vagina or the abnormal wetness that may come from an infection in the penis or vagina. Duct – Tube, the fallopian tubes may be called oviducts, because they are the path for an ovum. -

Development and Maintenance of Epidermal Stem Cells in Skin Adnexa
International Journal of Molecular Sciences Review Development and Maintenance of Epidermal Stem Cells in Skin Adnexa Jaroslav Mokry * and Rishikaysh Pisal Medical Faculty, Charles University, 500 03 Hradec Kralove, Czech Republic; [email protected] * Correspondence: [email protected] Received: 30 October 2020; Accepted: 18 December 2020; Published: 20 December 2020 Abstract: The skin surface is modified by numerous appendages. These structures arise from epithelial stem cells (SCs) through the induction of epidermal placodes as a result of local signalling interplay with mesenchymal cells based on the Wnt–(Dkk4)–Eda–Shh cascade. Slight modifications of the cascade, with the participation of antagonistic signalling, decide whether multipotent epidermal SCs develop in interfollicular epidermis, scales, hair/feather follicles, nails or skin glands. This review describes the roles of epidermal SCs in the development of skin adnexa and interfollicular epidermis, as well as their maintenance. Each skin structure arises from distinct pools of epidermal SCs that are harboured in specific but different niches that control SC behaviour. Such relationships explain differences in marker and gene expression patterns between particular SC subsets. The activity of well-compartmentalized epidermal SCs is orchestrated with that of other skin cells not only along the hair cycle but also in the course of skin regeneration following injury. This review highlights several membrane markers, cytoplasmic proteins and transcription factors associated with epidermal SCs. Keywords: stem cell; epidermal placode; skin adnexa; signalling; hair pigmentation; markers; keratins 1. Epidermal Stem Cells as Units of Development 1.1. Development of the Epidermis and Placode Formation The embryonic skin at very early stages of development is covered by a surface ectoderm that is a precursor to the epidermis and its multiple derivatives. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

A Comparative Study of the Ultrastructure of Microvilli in the Epithelium of Small and Large Intestine of Mice
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central A COMPARATIVE STUDY OF THE ULTRASTRUCTURE OF MICROVILLI IN THE EPITHELIUM OF SMALL AND LARGE INTESTINE OF MICE T. M. MUKHERJEE and A. WYNN WILLIAMS From the Electron Microscope Laboratory, the Departlnent of Pathology, the University of Otago Medical School, Dunedin, New Zealand ABSTRACT A comparative analysis of the fine structure of the microvilli on jejunal and colonic epi- thelial cells of the mouse intestine has been made. The microvilli in these two locations demonstrate a remarkably similar fine structure with respect to the thickness of the plasma membrane, the extent of the filament-free zone, and the characteristics of the microfila- ments situated within the microvillous core. Some of the core microfilaments appear to continue across the plasma membrane limiting the tip of the microvillus. The main differ- ence between the microvilli of small intestine and colon is in the extent and organization of the surface coat. In the small intestine, in addition to the commonly observed thin surface "fuzz," occasional areas of the jejunal villus show a more conspicuous surface coat covering the tips of the microvilli. Evidence has been put forward which indicates that the surface coat is an integral part of the epithelial cells. In contrast to the jejunal epithelium, the colonic epithelium is endowed with a thicker surface coat. Variations in the organization of the surface coat at different levels of the colonic crypts have also been noted. The func- tional significance of these variations in the surface coat is discussed. -

Molecular Signatures of Tissue-Specific
Developmental Cell Resource Molecular Signatures of Tissue-Specific Microvascular Endothelial Cell Heterogeneity in Organ Maintenance and Regeneration Daniel J. Nolan,1,6 Michael Ginsberg,1,6 Edo Israely,1 Brisa Palikuqi,1 Michael G. Poulos,1 Daylon James,1 Bi-Sen Ding,1 William Schachterle,1 Ying Liu,1 Zev Rosenwaks,2 Jason M. Butler,1 Jenny Xiang,4 Arash Rafii,1,7 Koji Shido,1 Sina Y. Rabbany,1,8 Olivier Elemento,3 and Shahin Rafii1,5,* 1Department of Genetic Medicine, Howard Hughes Medical Institute 2Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine 3HRH Prince Alwaleed Bin Talal Bin Abdulaziz Alsaud Institute for Computational Biomedicine 4Genomics Resource Core Facility Weill Cornell Medical College, New York, NY 10065, USA 5Ansary Stem Cell Institute, New York, NY 10065, USA 6Angiocrine Bioscience, New York, NY 10065, USA 7Weill Cornell Medical College-Qatar, Stem Cell and Microenvironment Laboratory, Education City, Qatar Foundation, Doha 24144, Qatar 8Bioengineering Program, Hofstra University, Hempstead, NY 11549, USA *Correspondence: srafi[email protected] http://dx.doi.org/10.1016/j.devcel.2013.06.017 SUMMARY been appreciated. Capillary ECs of the blood brain barrier (BBB) form a restrictive environment for passage between the Microvascular endothelial cells (ECs) within different brain tissue and the circulating blood. Many of the trafficking pro- tissues are endowed with distinct but as yet unrecog- cesses that are passive in other vascular beds are tightly nized structural, phenotypic, and functional attri- controlled in the brain (Rubin and Staddon, 1999). As opposed butes. We devised EC purification, cultivation, to the BBB, the capillary ECs of the kidney glomeruli are fenes- profiling, and transplantation models that establish trated for the filtration of the blood (Churg and Grishman, tissue-specific molecular libraries of ECs devoid of 1975). -

Diverse Repertoire of Human Adipocyte Subtypes Develops from Transcriptionally Distinct Mesenchymal Progenitor Cells
Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells So Yun Mina,b, Anand Desaia, Zinger Yanga,b, Agastya Sharmaa, Tiffany DeSouzaa, Ryan M. J. Gengaa,b,c, Alper Kucukurald, Lawrence M. Lifshitza, Søren Nielsene,f, Camilla Scheelee,f,g, René Maehra,c, Manuel Garbera,d, and Silvia Corveraa,1 aProgram in Molecular Medicine, University of Massachusetts Medical School, Worcester, MA 01655; bGraduate School of Biomedical Sciences, University of Massachusetts Medical School, Worcester, MA 01655; cDepartment of Medicine, Diabetes Center of Excellence, University of Massachusetts Medical School, Worcester, MA 01655; dProgram in Bioinformatics, University of Massachusetts Medical School, Worcester, MA 01655; eCentre of Inflammation and Metabolism, Rigshospitalet, University of Copenhagen, 1165 Copenhagen Denmark; fCentre for Physical Activity Research, Rigshospitalet, University of Copenhagen, 1165 Copenhagen Denmark; and gNovo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Science, University of Copenhagen, 1165 Copenhagen, Denmark Edited by Rana K. Gupta, University of Texas Southwestern Medical Center, Dallas, TX, and accepted by Editorial Board Member David J. Mangelsdorf July12, 2019 (received for review April 16, 2019) Single-cell sequencing technologies have revealed an unexpectedly UCP1 in response to stimulation. Lineage-tracing and gene- broad repertoire of cells required to mediate complex functions in expression studies point to distinct developmental origins for multicellular organisms. Despite the multiple roles of adipose tissue these adipocyte subtypes (5, 6). In adult humans, no specific depot + in maintaining systemic metabolic homeostasis, adipocytes are is solely composed of UCP1-containing adipocytes, but UCP-1 thought to be largely homogenous with only 2 major subtypes cells can be found interspersed within supraclavicular, para- recognized in humans so far. -

Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease Bone Marrow (Stem Cell) Transplant
Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease 1 Produced by St. Jude Children’s Research Hospital Departments of Hematology, Patient Education, and Biomedical Communications. Funds were provided by St. Jude Children’s Research Hospital, ALSAC, and a grant from the Plough Foundation. This document is not intended to take the place of the care and attention of your personal physician. Our goal is to promote active participation in your care and treatment by providing information and education. Questions about individual health concerns or specifi c treatment options should be discussed with your physician. For more general information on sickle cell disease, please visit our Web site at www.stjude.org/sicklecell. Copyright © 2009 St. Jude Children’s Research Hospital How did bone marrow (stem cell) transplants begin for children with sickle cell disease? Bone marrow (stem cell) transplants have been used for the treatment and cure of a variety of cancers, immune system diseases, and blood diseases for many years. Doctors in the United States and other countries have developed studies to treat children who have severe sickle cell disease with bone marrow (stem cell) transplants. How does a bone marrow (stem cell) transplant work? 2 In a person with sickle cell disease, the bone marrow produces red blood cells that contain hemoglobin S. This leads to the complications of sickle cell disease. • To prepare for a bone marrow (stem cell) transplant, strong medicines, called chemotherapy, are used to weaken or destroy the patient’s own bone marrow, stem cells, and infection fi ghting system. -

Expression of Integrins and Basement Membrane Components by Wound Keratinocytes
Expression of integrins and basement membrane components by wound keratinocytes. H Larjava, … , R H Kramer, J Heino J Clin Invest. 1993;92(3):1425-1435. https://doi.org/10.1172/JCI116719. Research Article Extracellular matrix proteins and their cellular receptors, integrins, play a fundamental role in keratinocyte adhesion and migration. During wound healing, keratinocytes detach, migrate until the two epithelial sheets confront, and then regenerate the basement membrane. We examined the expression of different integrins and their putative ligands in keratinocytes during human mucosal wound healing. Migrating keratinocytes continuously expressed kalinin but not the other typical components of the basement membrane zone: type IV collagen, laminin, and type VII collagen. When the epithelial sheets confronted each other, these missing basement membrane components started to appear gradually through the entire wound area. The expression of integrin beta 1 subunit was increased in keratinocytes during migration. The beta 1-associated alpha 2 and alpha 3 subunits were expressed constantly by wound keratinocytes whereas the alpha 5 subunit was present only in keratinocytes during reepithelialization. Furthermore, migrating cells started to express alpha v-integrins which were not present in the nonaffected epithelium. All keratinocytes also expressed the alpha 6 beta 4 integrin during migration. In the migrating cells, the distribution of integrins was altered. In normal mucosa, beta 1-integrins were located mainly on the lateral plasma membrane and alpha 6 beta 4 at the basal surface of basal keratinocytes in the nonaffected tissue. In wounds, integrins were found in filopodia of migrating keratinocytes, and also surrounding cells in […] Find the latest version: https://jci.me/116719/pdf Expression of Integrins and Basement Membrane Components by Wound Keratinocytes Hannu Lariava, * Tuula Salo,t Kirsi Haapasalmi, * Randall H. -

Corrective Gene Transfer of Keratinocytes from Patients with Junctional Epidermolysis Bullosa Restores Assembly of Hemidesmosomes in Reconstructed Epithelia
Gene Therapy (1998) 5, 1322–1332 1998 Stockton Press All rights reserved 0969-7128/98 $12.00 http://www.stockton-press.co.uk/gt Corrective gene transfer of keratinocytes from patients with junctional epidermolysis bullosa restores assembly of hemidesmosomes in reconstructed epithelia J Vailly1, L Gagnoux-Palacios1, E Dell’Ambra2, C Rome´ro1, M Pinola3, G Zambruno3, M De Luca2,3 J-P Ortonne1,4 and G Meneguzzi1 1U385 INSERM, Faculte´ de Me´decine, Nice; 4Service de Dermatologie, Hoˆpital L’Archet, Nice, France; Laboratories of 2Tissue Engineering and 3Molecular and Cell Biology, Istituto Dermopatico dell’Immacolata, Rome, Italy Herlitz junctional epidermolysis bullosa (H-JEB) provides deposited into the extracellular matrix. Re-expression of a promising model for somatic gene therapy of heritable laminin-5 induced cell spreading, nucleation of hemides- mechano-bullous disorders. This genodermatosis is mosomal-like structures and enhanced adhesion to the cul- caused by the lack of laminin-5 that results in absence of ture substrate. Organotypic cultures performed with the hemidesmosomes (HD) and defective adhesion of squam- transduced keratinocytes, reconstituted epidermis closely ous epithelia. To establish whether re-expression of lami- adhering to the mesenchyme and presenting mature hemi- nin-5 can restore assembly of the dermal-epidermal attach- desmosomes, bridging the cytoplasmic intermediate fila- ment structures lacking in the H-JEB skin, we corrected the ments of the basal cells to the anchoring filaments of the genetic mutation hindering expression of the 3 chain of basement membrane. Our results provide the first evi- laminin-5 in human H-JEB keratinocytes by transfer of a dence of phenotypic reversion of JEB keratinocytes by laminin 3 transgene. -

Adaptive Immune Systems
Immunology 101 (for the Non-Immunologist) Abhinav Deol, MD Assistant Professor of Oncology Wayne State University/ Karmanos Cancer Institute, Detroit MI Presentation originally prepared and presented by Stephen Shiao MD, PhD Department of Radiation Oncology Cedars-Sinai Medical Center Disclosures Bristol-Myers Squibb – Contracted Research What is the immune system? A network of proteins, cells, tissues and organs all coordinated for one purpose: to defend one organism from another It is an infinitely adaptable system to combat the complex and endless variety of pathogens it must address Outline Structure of the immune system Anatomy of an immune response Role of the immune system in disease: infection, cancer and autoimmunity Organs of the Immune System Major organs of the immune system 1. Bone marrow – production of immune cells 2. Thymus – education of immune cells 3. Lymph Nodes – where an immune response is produced 4. Spleen – dual role for immune responses (especially antibody production) and cell recycling Origins of the Immune System B-Cell B-Cell Self-Renewing Common Progenitor Natural Killer Lymphoid Cell Progenitor Thymic T-Cell Selection Hematopoetic T-Cell Stem Cell Progenitor Dendritic Cell Myeloid Progenitor Granulocyte/M Macrophage onocyte Progenitor The Immune Response: The Art of War “Know your enemy and know yourself and you can fight a hundred battles without disaster.” -Sun Tzu, The Art of War Immunity: Two Systems and Their Key Players Adaptive Immunity Innate Immunity Dendritic cells (DC) B cells Phagocytes (Macrophages, Neutrophils) Natural Killer (NK) Cells T cells Dendritic Cells: “Commanders-in-Chief” • Function: Serve as the gateway between the innate and adaptive immune systems. -

Bone Marrow Biopsy
Helpline (freephone) 0808 808 5555 [email protected] www.lymphoma-action.org.uk Bone marrow biopsy This information is about a test called a bone marrow biopsy. You might have one to check if you have lymphoma in your bone marrow. On this page What is bone marrow? What is a bone marrow biopsy? Who might need one? Having a bone marrow biopsy Is a bone marrow biopsy safe? Getting the results We have separate information about the topics in bold font. Please get in touch if you’d like to request copies or if you would like further information about any aspect of lymphoma. Phone 0808 808 5555 or email [email protected]. What is bone marrow? Bone marrow is the spongy tissue in the middle of some of the bigger bones in your body, such as your thigh bone (femur), breastbone (sternum), hip bone (pelvis) and back bones (vertebrae). Your bone marrow is where blood cells are made. It contains cells called blood (‘haemopoietic’) stem cells. Stem cells are undeveloped cells that can divide and grow into all the blood cells you need. This includes red blood cells, platelets and all the different types of white blood cells. Page 1 of 8 © Lymphoma Action Figure: The different blood cells that develop in the bone marrow What is a bone marrow biopsy? A bone marrow biopsy is a test that involves taking a sample of bone marrow to be examined under a microscope. The samples are sent to a lab where an expert examines them. -

Endotrophin Triggers Adipose Tissue Fibrosis and Metabolic Dysfunction
ARTICLE Received 12 Sep 2013 | Accepted 21 Feb 2014 | Published 19 Mar 2014 DOI: 10.1038/ncomms4485 Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction Kai Sun1,*, Jiyoung Park1,2,*, Olga T. Gupta1, William L. Holland1, Pernille Auerbach3, Ningyan Zhang4, Roberta Goncalves Marangoni5, Sarah M. Nicoloro6, Michael P. Czech6, John Varga5, Thorkil Ploug3, Zhiqiang An4 & Philipp E. Scherer1,7 We recently identified endotrophin as an adipokine with potent tumour-promoting effects. However, the direct effects of local accumulation of endotrophin in adipose tissue have not yet been studied. Here we use a doxycycline-inducible adipocyte-specific endotrophin overexpression model to demonstrate that endotrophin plays a pivotal role in shaping a metabolically unfavourable microenvironment in adipose tissue during consumption of a high-fat diet (HFD). Endotrophin serves as a powerful co-stimulator of pathologically relevant pathways within the ‘unhealthy’ adipose tissue milieu, triggering fibrosis and inflammation and ultimately leading to enhanced insulin resistance. We further demonstrate that blocking endotrophin with a neutralizing antibody ameliorates metabolically adverse effects and effectively reverses metabolic dysfunction induced during HFD exposure. Collectively, our findings demonstrate that endotrophin exerts a major influence in adipose tissue, eventually resulting in systemic elevation of pro-inflammatory cytokines and insulin resistance, and the results establish endotrophin as a potential target in the context of metabolism and cancer. 1 Touchstone Diabetes Center, Department of Internal Medicine, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, Texas 75390, USA. 2 Department of Biological Sciences, School of Life Sciences, Ulsan National Institute of Science and Technology, 50 UNIST street, Ulsan 689-798, Korea.