Considering Contact Lens CORNEAL RESHAPING
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

To See the Invisible: the Quest of Imaging Vitreous J
DOP42005.qxd 4/15/08 11:34 AM Page 5 Meyer CH (ed): Vital Dyes in Vitreoretinal Surgery. Dev Ophthalmol. Basel, Karger, 2008, vol 42, pp 5–28 To See the Invisible: The Quest of Imaging Vitreous J. Sebag VMR Institute, University of Southern California, Los Angeles, Calif., USA Abstract Purpose: Imaging vitreous has long been a quest to view what is, by design, invisible. This chapter will review important historical aspects, past and present imaging methodologies, and new technologies that are currently in development for future research and clinical applications. Methods: Classic and modern histologic techniques, dark-field slit microscopy, clinical slit lamp biomicroscopy, standard and scanning laser ophthalmoscopy (SLO), ultrasonography, optical coherence tomography (OCT), com- bined OCT-SLO, magnetic resonance and Raman spectroscopies, and dynamic light scattering method- ologies are presented. Results: The best available histologic techniques for imaging vitreous are those that avoid rapid dehydration of vitreous specimens. Dark-field slit microscopy enables in vitro imaging without dehydration or tissue fixatives. OCT enables better in vivo visualization of the vitreoretinal inter- face than SLO and ultrasonography, but does not adequately image the vitreous body. The combination of OCT with SLO has provided useful new imaging capabilities, but only at the vitreoretinal interface. Dynamic light scattering can evaluate the vitreous body by determining the average sizes of vitreous macromolecules in aging, disease, and as a means to assess the effects of pharmacologic vitreolysis. Raman spectroscopy can detect altered vitreous molecules, such as glycated collagen and other pro- teins in diabetic vitreopathy and possibly other diseases. Conclusions: A better understanding of normal vitreous physiology and structure and how these change in aging and disease is needed to develop more effective therapies and prevention. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Topic 3: Operation of Simple Lens
V N I E R U S E I T H Y Modern Optics T O H F G E R D I N B U Topic 3: Operation of Simple Lens Aim: Covers imaging of simple lens using Fresnel Diffraction, resolu- tion limits and basics of aberrations theory. Contents: 1. Phase and Pupil Functions of a lens 2. Image of Axial Point 3. Example of Round Lens 4. Diffraction limit of lens 5. Defocus 6. The Strehl Limit 7. Other Aberrations PTIC D O S G IE R L O P U P P A D E S C P I A S Properties of a Lens -1- Autumn Term R Y TM H ENT of P V N I E R U S E I T H Y Modern Optics T O H F G E R D I N B U Ray Model Simple Ray Optics gives f Image Object u v Imaging properties of 1 1 1 + = u v f The focal length is given by 1 1 1 = (n − 1) + f R1 R2 For Infinite object Phase Shift Ray Optics gives Delta Fn f Lens introduces a path length difference, or PHASE SHIFT. PTIC D O S G IE R L O P U P P A D E S C P I A S Properties of a Lens -2- Autumn Term R Y TM H ENT of P V N I E R U S E I T H Y Modern Optics T O H F G E R D I N B U Phase Function of a Lens δ1 δ2 h R2 R1 n P0 P ∆ 1 With NO lens, Phase Shift between , P0 ! P1 is 2p F = kD where k = l with lens in place, at distance h from optical, F = k0d1 + d2 +n(D − d1 − d2)1 Air Glass @ A which can be arranged to|giv{ze } | {z } F = knD − k(n − 1)(d1 + d2) where d1 and d2 depend on h, the ray height. -

The Camera Versus the Human Eye
The Camera Versus the Human Eye Nov 17, 2012 ∙ Roger Cicala This article was originally published as a blog. Permission was granted by Roger Cicala to re‐ publish the article on the CTI website. It is an excellent article for those police departments considering the use of cameras. This article started after I followed an online discussion about whether a 35mm or a 50mm lens on a full frame camera gives the equivalent field of view to normal human vision. This particular discussion immediately delved into the optical physics of the eye as a camera and lens — an understandable comparison since the eye consists of a front element (the cornea), an aperture ring (the iris and pupil), a lens, and a sensor (the retina). Despite all the impressive mathematics thrown back and forth regarding the optical physics of the eyeball, the discussion didn’t quite seem to make sense logically, so I did a lot of reading of my own on the topic. There won’t be any direct benefit from this article that will let you run out and take better photographs, but you might find it interesting. You may also find it incredibly boring, so I’ll give you my conclusion first, in the form of two quotes from Garry Winogrand: A photograph is the illusion of a literal description of how the camera ‘saw’ a piece of time and space. Photography is not about the thing photographed. It is about how that thing looks photographed. Basically in doing all this research about how the human eye is like a camera, what I really learned is how human vision is not like a photograph. -
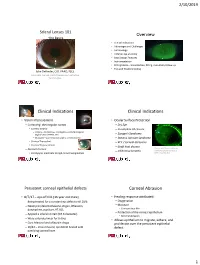
Scleral Lens Fitting the Basics
2/10/2019 Scleral Lenses 101 Overview -the basics • Clinical Indications • Advantages and Challenges • Terminology • Anterior eye anatomy • Basic Design Features • Instrumentation • Fitting basics – lens selection, fitting, evaluation, follow-up • Tips and Troubleshooting Julie DeKinder, O.D. FAAO, FSLS Diplomate, Cornea, Contact Lenses and Refractive Technologies Clinical Indications Clinical Indications • Vision Improvement • Ocular Surface Protection – Correcting the irregular cornea – Dry Eye • Corneal Ectasia – Incomplete lid closure – Primary – Keratoconus, Keratoglobus, Pellucid marginal degeneration (INTACS, CXL) – Sjorgen’s Syndrome – Secondary – post-refractive surgery, corneal trauma – Stevens-Johnson Syndrome • Corneal Transplant – RCE / corneal abrasions • Corneal Degenerations – Graft host disease – Normal Cornea Patient with Steven s-Johnson – Infiltrative keratitis Syndrome; photo courtesy of • Presbyopia, moderate to high corneal astigmatism Beth Kinoshita, O.D. Persistent corneal epithelial defects Corneal Abrasion • 8/7/17 – epi-off CXL (16 year old male) • Healing response attributed: – Being treated for a constant epi defect until 10/6 – Oxygenation – Neomycin/dexamethasone, Zirgan, Oflaxacin, – Moisture doxycycline, acyclovir, AT, BCL • Constant tear film – Protection of the corneal epithelium – Applied a scleral contact (15.6 diameter) • Minimal abrasion – Wore extended wear for 6 days • Allows epithelium to migrate, adhere, and – Cont Maxitrol and oflaxacin drops proliferate over the persistent epithelial – 10/12 -

Unit 4 Anatomy of Lens
UNIT 4 ANATOMY OF LENS Structure 4.0 Objectives 4.1 Introduction 4.2 Anatomy of Lens 4.3 Let Us Surri Up 4.4 Answers to Check Your Progress After reading this unit, you should be able to iulderstancl: @ different parts of the lens; and e .functions of lens. 4,1 INTRODUCTION I In the previous unit you have studied the anatomy of outer coats of eyeball and anatomy of anterior and posterior chamber. Lens ,is a structure that occupies space in posterior chamber. In this unit you will study the structure and composition of crystalline lens. Lens is a transparent structure of the eye. It is a:biconvex structure and its main functioil is to focus light rays on the retina. Lens also helps. in the process of accommodation. As age increases transparency of lens is lost. This process is called cataru.act. Cataract is a co~niiondisorder of eye. 4.2 ANATOMY OF LENS ,'. Lens is a biconvex rans spa rent sln~cturkof the eye. Along with cornea, lens forms the optical surfaces of the eye and helps in the fo'omlation of iinage on the retina. Lens acts as a convex lens and when light falls on it, light is converged. Lens has to be trai~spxuntso as to liinction as an eflective optical system. When transparency of lcils is lost this colldition is known as cataract. -I Descr!'plion of Lens Shape: Biconvex Situation: Between iris anteriorly and vitreous posteriorly. It lies wilhiil the anterior concavity of vitreous which is known as patellar fossa. Pole of Lens: The centre of anterior and posterior surface is laown as pole of the id g ,I lens. -

The Official Guide to Scleral Lens Terminology
Contact Lens and Anterior Eye xxx (xxxx) xxx–xxx Contents lists available at ScienceDirect Contact Lens and Anterior Eye journal homepage: www.elsevier.com/locate/clae The official guide to scleral lens terminology ⁎ Langis Michaudd, , Michael Lipsonc, Elise Kramera, Maria Walkerb a private practice, United States b University of Houston, United States c University of Michigan, United States d Universite de Montreal, Canada ARTICLE INFO ABSTRACT Keywords: Purpose: In absence of scleral lens standards, this article aims to provide an official definition of terms related to Scleral lenses scleral lens fitting and manufacturing, in order to make more uniform the use of appropriate termswhende- Terminology scribing, writing or lecturing about scelral lenses. Adoption of a common terminology may also favor more Lens design fruitful exchanges between eyecare practitioners and manufacturers. Lens manufacturing Methods: A committee of 12 advances scleral lens clinicians met and develop a list of terms related to scleral lens Sagittal heigth fit and manufacturing. Litterature review was made using PubMed database with the keywords “scleral lenses” and “terminology”. Other related publications such as textbooks were also considered valid references. Validation of the terms selected and their suggested definition was made by consultation of other experts inthe field, over 2 years. A final version was adopted by the Scleral Lens Education Society latein2018. Results: This article contains three main sections. Section I provides the definition of a scleral lens. Section II addresses the general terminology habitually applied to contact lens field but in the context of scleral lens usage. Finally, Section III suggests a decription of terms specifically used when fitting or manufacturing scleral lenses. -
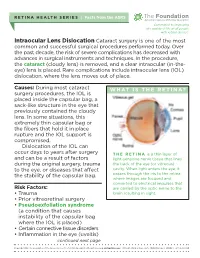
Intraocular Lens Dislocation Cataract Surgery Is One of the Most Common and Successful Surgical ProCedures Performed Today
RETINA HEALTH SERIES | Facts from the ASRS The Foundation American Society of Retina Specialists Committed to improving the quality of life of all people with retinal disease. Intraocular Lens Dislocation Cataract surgery is one of the most common and successful surgical pro cedures performed today. Over the past decade, the risk of severe complications has decreased with advances in surgical instruments and techniques. In the procedure, the cataract (cloudy lens) is removed, and a clear intraocular (in-the- eye) lens is placed. Rare complications include intraocular lens (IOL) dislocation, where the lens moves out of place. Causes: During most cataract WHAT IS THE RETINA? surgery pro cedures, the IOL is placed inside the capsular bag, a sack-like structure in the eye that previously contained the cloudy lens. In some situations, this extremely thin capsular bag or the fibers that hold it in place rupture and the IOL support is compromised. Dislocation of the IOL can occur days to years after surgery THE RETINA is a thin layer of and can be a result of factors light-sensitive nerve tissue that lines during the original surgery, trauma the back of the eye (or vitreous) to the eye, or diseases that affect cavity. When light enters the eye, it the stability of the capsular bag. passes through the iris to the retina where images are focused and converted to electrical impulses that Risk Factors: are carried by the optic nerve to the • Trauma brain resulting in sight. • Prior vitreoretinal surgery • Pseudoexfoliation syndrome (a condition that causes instability of the capsular bag where the IOL is placed) • Certain connective tissue disorders • Inflammation in the eye (uveitis) continued next page Copyright 2016 The Foundation of the American Society of Retina Specialists. -

Cataracts: What Are They? the Crystalline Lens
Cataracts: what are they? A cataract is a clouding of the normally clear lens of your eye. Most cataracts are related to ageing and therefore are very common in older people. The density of a cataract can range from a mild clouding that isn’t noticed at all to a visually blinding opaque lens. It is normal to see a small degree of cataract in most eyes over the age of 60. When the vision impairment caused by the cataract begins to inconvenience your normal lifestyle, you may need cataract removal surgery. The surgery is in most cases a safe and effective procedure. The crystalline lens The crystalline lens of the eye is a natural lens which produces one third of the eye’s total optical power and focuses light into an image on the retina (the light-sensitive tissue at the back of the eye). The crystalline lens is elastic which allows it to flex in order to change its shape. The act of changing shape causes the lens to change power in order to focus on objects at close range. At birth, the lens contains elongated cells which resemble fibres. They are completely transparent due to their highly ordered arrangement and along with other protein and chemical processes this keeps the lens transparent. With age, additional fibres are added to the outside, wrapping around the inner portion of the lens (the nucleus). The outer portion of the lens is termed the cortex. This structure is similar to the layers of an onion. Lens A sharp, clear image is formed on the retina when light passes through the normally transparent lens. -

Pathology of the Lens
Pathology of the lens Carol Naranjo, LV, DACVP, DECVP, PhD IDEXX Laboratories Embryonal development Gelatt’s Veterinary Ophthalmology, 5th Ed. Normal histology • Lens capsule – Anterior > posterior • Lens cortex – Lens epithelium and lens bow • Lens nucleus • Artifact! Lens bow Anterior lens capsule Posterior lens capsule Congenital conditions • Aphakia • Microphakia • Lens coloboma Dr. Dubielzig (COPLOW) • Spherophakia • Lenticonus/lentiglobus Lenticonus Courtesy of Dr. Dubielzig (COPLOW) Cataract • Any opacification of the lens • Various classifications • Etiology: – Senile, hereditary, diabetic, toxic – Secondary: intraocular inflam, retinal degeneration, glaucoma, neoplasia • Extension of lens involvement – Some variation b/w clinical-pathological assessment • Location Congenital cataract • Abnormal position or lysis of the nucleus. • Dysplastic changes in the lens capsule – Duplication, wrinkling • Posterior migration of lens epithelium • Fetal vasculature anomalies Congenital cataract Courtesy of Dr. Dubielzig (COPLOW) Cataract - location • Subcapsular – anterior: – Proliferation of LEC – LEC fibrous metaplasia – Collagenous mb Cataract - location • Subcapsular – posterior: – Migration of lens epithelium – Proliferation, fibrous metaplasia, collagen membranes Cataract - location • Cortical: – Early/incipient: not always detected – Mature: • Bladder cells • Morgagnian globules – Intumescent: • Lens swelling • Morgagnian globules throughout the cortex Intumesent cataract Courtesy of Dr. Dubielzig (COPLOW) Cataract - location • Cortical: -

Human Eye • Human Eye Is a Simple Single Lens System • Crystalline Lens
Human Eye • Human eye is a simple single lens system • Crystalline lens provide focus • Cornea: outer surface protection • Iris: control light • Retina: where image is focused • Note images are inverted Human Eye Distance • Crystalline lens to retina distance 24.4 mm • Eye focuses object up to 25 cm from it • Called the near point or Dv = 25 cm Magnification of Lens • Lateral change in distance equals change in image size • Measures change in apparent image size y′ s′ m = M = = − y s Magnification with Index Change • Many different ways of measuring magnification • With curved index of refraction surface measure apparent change in distance to image • Called Lateral Magnification s′ − r m = − s + r • m is + if image virtual, - if real Angular Magnification • For the eye look at angular magnification θ ′ m = M = θ • Represents the change in apparent angular size Simple Magnifying Glass • Human eye focuses near point or Dv = 25 cm • Magnification of object: ratio of angles at eye between unaided and lens • Angle of Object with lens y y tan(θ≈) = = θ Dv 25 • For maximum magnification place object at lens f (in cm) y θ ′ = f • Thus magnification is (where f in cm) θ ′ 25 m = = θ f • e.g. What is the magnification of a lens f = 1 inch = 2.5 cm θ ′ 25 25 m = = = = 10 θ f 2.5 Power of a Lens or Surface • Power: measures the ability to create converging/diverging light by a lens • Measured in Diopters (D) or 1/m • For a simple curved surface n′ − n P = r • For a thin lens 1 P = f • Converging lens have + D, diverging - D • eg f = 50 cm, D = +2