Advances in Regeneration of Retinal Ganglion Cells and Optic Nerves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neuroinflammation Triggered by Β-Glucan/Dectin-1 Signaling Enables CNS Axon Regeneration
Neuroinflammation triggered by β-glucan/dectin-1 signaling enables CNS axon regeneration Katherine T. Baldwina,b,1, Kevin S. Carbajalc,d,1, Benjamin M. Segalc,d,2, and Roman J. Gigera,b,c,d,2 aDepartment of Cell and Developmental Biology, bCellular and Molecular Biology Graduate Program, cNeuroscience Graduate Program, and dHoltom-Garrett Program in Neuroimmunology, Department of Neurology, University of Michigan School of Medicine, Ann Arbor, MI 48109 Edited by Ben A. Barres, Stanford University School of Medicine, Stanford, CA, and approved January 22, 2015 (received for review December 22, 2014) Innate immunity can facilitate nervous system regeneration, yet growth can be undermined by concurrent toxicity (9). A deeper the underlying cellular and molecular mechanisms are not well understanding of these opposing effects will be important for understood. Here we show that intraocular injection of lipopoly- exploiting immunomodulatory pathways to promote neural re- saccharide (LPS), a bacterial cell wall component, or the fungal cell pair while minimizing bystander damage. wall extract zymosan both lead to rapid and comparable intravi- In the present study, we investigated the pathways that drive treal accumulation of blood-derived myeloid cells. However, when innate immune-mediated axon regeneration after ONC. We in- combined with retro-orbital optic nerve crush injury, lengthy duced sterile inflammation in the vitreous on the day of injury by growth of severed retinal ganglion cell (RGC) axons occurs only i.o. administration of zymosan or constituents of zymosan clas- in zymosan-injected mice, and not in LPS-injected mice. In mice sified as pathogen-associated molecular patterns (PAMPs). PAMPs dectin-1 deficient for the pattern recognition receptor but not are highly conserved microbial structures that serve as ligands for TLR2 , Toll-like receptor-2 ( ) zymosan-mediated RGC regeneration pattern recognition receptors (PRRs). -

Symptoms of Age Related Macular Degeneration
WHAT IS MACULAR DEGENERATION? wavy or crooked, visual distortions, doorway and the choroid are interrupted causing waste or street signs seem bowed, or objects may deposits to form. Lacking proper nutrients, the light- Age related macular degeneration (AMD) is appear smaller or farther away than they sensitive cells of the macula become damaged. a disease that may either suddenly or gradually should, decrease in or loss of central vision, and The damaged cells can no longer send normal destroy the macula’s ability to maintain sharp, a central blurry spot. signals from the macula through the optic nerve to central vision. Interestingly, one’s peripheral or DRY: Progression with dry AMD is typically slower your brain, and consequently your vision becomes side vision remains unaffected. AMD is the leading de-gradation of central vision: need for increasingly blurred cause of “legal blindness” in the United States for bright illumination for reading or near work, diffi culty In either form of AMD, your vision may remain fi ne persons over 65 years of age. AMD is present in adapting to low levels of illumination, worsening blur in one eye up to several years even while the other approximately 10 percent of the population over of printed words, decreased intensity or brightness of eye’s vision has degraded. Most patients don’t the age of 52 and in up to 33 percent of individuals colors, diffi culty recognizing faces, gradual increase realize that one eye’s vision has been severely older than 75. The macula allows alone gives us the in the haziness of overall vision, and a profound drop reduced because your brain compensates the bad ability to have: sharp vision, clear vision, color vision, in your central vision acuity. -

How Clean Is Your Capsule?
Eye (1989) 3, 678-684 How Clean is Your Capsule? W. T. GREEN and D. L. BOASE Portsmouth Summary Proliferation of residual lens epithelial cells is believed to be the major cause of pos terior capsule opacification following extracapsular cataract extraction. During sur gery these cells can be visualised with appropriate illumination facilitating their mechanical removal with the McIntyre cannula. When flat preparations of the anterior capsule are examined by light microscopy, the areas 'cleaned' of cells in this way appear transparent but scanning electron microscopy reveals tufts of remaining debris which may represent points of cellular attachment to the capsule. Control of lens epithelial cell proliferation is important for the future development of cataract surgery. The undoubted advantages of extracapsular and also on human cadaver eyes. A horizontal cataract extraction are offset in many patients capsulotomy in the upper part of the lens by posterior capsule opacification requiring allowed nucleus removal. Irrigation and caps ulotomy. Not only is this disappointing aspiration of the cortical lens material was for the patient, but the procedure carries a then carried out using a McIntyre cannula risk of serious complications. with Hartman's irrigation solution. During in The major cause of posterior capsule opac vitro surgery this was aided by first removing ification is proliferation of residual lens epi the entire cornea and iris to improve visual thelial cells. I If these cells could be removed at isation and explore different methods of the time of surgery we believe that the inci illumination. dence of posterior capsule opacification and The importance of illumination was first the need for subsequent capsulotomy would suspected when it was observed, during rou be reduced. -

Epha Receptors and Ephrin-A Ligands Are Upregulated by Monocytic
Mukai et al. BMC Cell Biology (2017) 18:28 DOI 10.1186/s12860-017-0144-x RESEARCHARTICLE Open Access EphA receptors and ephrin-A ligands are upregulated by monocytic differentiation/ maturation and promote cell adhesion and protrusion formation in HL60 monocytes Midori Mukai, Norihiko Suruga, Noritaka Saeki and Kazushige Ogawa* Abstract Background: Eph signaling is known to induce contrasting cell behaviors such as promoting and inhibiting cell adhesion/ spreading by altering F-actin organization and influencing integrin activities. We have previously demonstrated that EphA2 stimulation by ephrin-A1 promotes cell adhesion through interaction with integrins and integrin ligands in two monocyte/ macrophage cell lines. Although mature mononuclear leukocytes express several members of the EphA/ephrin-A subclass, their expression has not been examined in monocytes undergoing during differentiation and maturation. Results: Using RT-PCR, we have shown that EphA2, ephrin-A1, and ephrin-A2 expression was upregulated in murine bone marrow mononuclear cells during monocyte maturation. Moreover, EphA2 and EphA4 expression was induced, and ephrin-A4 expression was upregulated, in a human promyelocytic leukemia cell line, HL60, along with monocyte differentiation toward the classical CD14++CD16− monocyte subset. Using RT-PCR and flow cytometry, we have also shown that expression levels of αL, αM, αX, and β2 integrin subunits were upregulated in HL60 cells along with monocyte differentiation while those of α4, α5, α6, and β1 subunits were unchanged. Using a cell attachment stripe assay, we have shown that stimulation by EphA as well as ephrin-A, likely promoted adhesion to an integrin ligand- coated surface in HL60 monocytes. Moreover, EphA and ephrin-A stimulation likely promoted the formation of protrusions in HL60 monocytes. -

Selective Attention Within the Foveola
ARTICLES Selective attention within the foveola Martina Poletti1 , Michele Rucci1,2 & Marisa Carrasco3,4 Efficient control of attentional resources and high-acuity vision are both fundamental for survival. Shifts in visual attention are known to covertly enhance processing at locations away from the center of gaze, where visual resolution is low. It is unknown, however, whether selective spatial attention operates where the observer is already looking—that is, within the high-acuity foveola, the small yet disproportionally important rod-free region of the retina. Using new methods for precisely controlling retinal stimulation, here we show that covert attention flexibly improves and speeds up both detection and discrimination at loci only a fraction of a degree apart within the foveola. These findings reveal a surprisingly precise control of attention and its involvement in fine spatial vision. They show that the commonly studied covert shifts of attention away from the fovea are the expression of a global mechanism that exerts its action across the entire visual field. Covert attention is essential for visual perception. Among its many previous studies. We then investigated the consequences of attention advantages, covert allocation of attentional resources increases con- for both detection (experiment 2) and discrimination (experiments trast sensitivity and spatial resolution, speeds information accrual and 3 and 4) tasks within the foveola. reaction times1–4, and alters the signal at the target location during saccade preparation5–7. Covert attention has been studied sometimes RESULTS in the parafovea (1°–5°) and mostly in the perifovea (5°–10°) and Experiment 1 consisted of a central spatial cueing task with para- periphery (>10° of eccentricity)—that is, far outside the foveola, the foveal stimuli (Fig. -

Specification of Optic Nerve Oligodendrocyte Precursors by Retinal Ganglion Cell Axons
The Journal of Neuroscience, July 19, 2006 • 26(29):7619–7628 • 7619 Cellular/Molecular Specification of Optic Nerve Oligodendrocyte Precursors by Retinal Ganglion Cell Axons Limin Gao and Robert H. Miller Department of Neurosciences, Case Western Reserve University, Cleveland, Ohio 44106 Cell fate commitment in the developing CNS frequently depends on localized cell–cell interactions. In the avian visual system the optic nerve oligodendrocytes are derived from founder cells located at the floor of the third ventricle. Here we show that the induction of these founder cells is directly dependent on signaling from the retinal ganglion cell (RGC) axons. The appearance of oligodendrocyte precursor cells (OPCs) correlates with the projection of RGC axons, and early eye removal dramatically reduces the number of OPCs. In vitro signaling from retinal neurites induces OPCs in responsive tissue. Retinal axon induction of OPCs is dependent on sonic hedgehog (Shh) andneuregulinsignaling,andtheinhibitionofeithersignalreducesOPCinductioninvivoandinvitro.ThedependenceofOPCsonretinal axonal cues appears to be a common phenomenon, because ocular retardation (orJ) mice lacking optic nerve have dramatically reduced OPCs in the midline of the third ventricle. Key words: oligodendrocyte precursors; optic nerve; axon induction; sonic hedgehog; neuregulin; retinal ganglion cells Introduction contributes to the specification of ventral midline cells (Dale et al., During development the oligodendrocytes, the myelinating cells 1997); however, OPCs arise later -

Early Neuronal Processes Interact with Glia to Establish a Scaffold For
bioRxiv preprint doi: https://doi.org/10.1101/754416; this version posted August 31, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Early neuronal processes interact with glia to establish a scaffold for orderly innervation of the cochlea Running Title: Glia and early cochlear wiring N. R. Druckenbrod1, E. B. Hale, O. O. Olukoya, W. E. Shatzer, and L.V. Goodrich* Department of Neurobiology, Harvard Medical School, Boston, MA, 02115 1Current address: Decibel Therapeutics, Boston, Ma, 02215 *correspondence should be addressed to [email protected] Keywords: neuron-glia interactions, axon guidance, spiral ganglion neuron, cochlea Authors’ contributions: This study was conceived of and designed by NRD and LVG. NRD, EBH, OOO, and WES performed experiments and analyzed results. LVG analyzed results and wrote the manuscript. bioRxiv preprint doi: https://doi.org/10.1101/754416; this version posted August 31, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Summary: Although the basic principles of axon guidance are well established, it remains unclear how axons navigate with high fidelity through the complex cellular terrains that are encountered in vivo. To learn more about the cellular strategies underlying axon guidance in vivo, we analyzed the developing cochlea, where spiral ganglion neurons extend processes through a heterogeneous cellular environment to form tonotopically ordered connections with hair cells. -

Retinal Ganglion Cell Loss Is Size Dependent in Experimental Glaucoma
Investigative Ophthalmology & Visual Science, Vol. 32, No. 3, March 1991 Copyright © Association for Research in Vision and Ophthalmology Retinal Ganglion Cell Loss Is Size Dependent in Experimental Glaucoma Yoseph Glovinsky,* Harry A. Quigley,f and Gregory R. Dunkelbergerf Thirty-two areas located in the temporal midperipheral retina were evaluated in whole-mount prepara- tions from four monkeys with monocular experimental glaucoma. Diameter frequency distributions of remaining ganglion cells in the glaucomatous eye were compared with corresponding areas in the normal fellow eye. Large cells were significantly more vulnerable at each stage of cell damage as determined by linear-regression analysis. The magnitude of size-dependent loss was moderate at an early stage (20% loss), peaked at 50% total cell loss, and decreased in advanced damage (70% loss). In glaucomatous eyes, the lower retina had significantly more large cell loss than the corresponding areas of the upper retina. In optic nerve zones that matched the retinal areas studied, large axons selectively were damaged first. Psychophysical testing aimed at functions subserved by larger ganglion cells is recommended for detection and follow-up of early glaucoma; however, assessment of functions unique to small cells is more appropriate for detecting change in advanced glaucoma. Invest Ophthalmol Vis Sci 32:484-491, 1991 Current psychophysical tests do not detect glau- tage of ideal cellular preservation. Eyes with mild, comatous damage until a substantial minority of reti- moderate, and late damage were evaluated. In addi- nal ganglion cells have died.1'2 To develop more sen- tion, we correlated the damage patterns in the retinas sitive tests, a comprehensive understanding of the and optic nerves of the glaucomatous eyes. -

Crosstalk Between Integrin and Receptor Tyrosine Kinase Signaling in Breast Carcinoma Progression
BMB reports Mini Review Crosstalk between integrin and receptor tyrosine kinase signaling in breast carcinoma progression Young Hwa Soung, John L. Clifford & Jun Chung* Department of Biochemistry and Molecular Biology, Louisiana State University Health Sciences Center, Shreveport, Louisiana 71130 This review explored the mechanism of breast carcinoma pro- cancer originates from breast epithelial cells that are trans- gression by focusing on integrins and receptor tyrosine kinases formed into metastatic carcinomas. Metastatic potential and re- (or growth factor receptors). While the primary role of integrins sponsiveness to treatment vary depending on the expression of was previously thought to be solely as mediators of adhesive hormone receptors such as estrogen receptor and progesterone interactions between cells and extracellular matrices, it is now receptor (5), RTKs such as ErbB-2, epidermal growth factor re- believed that integrins also regulate signaling pathways that ceptor (EGFR), and hepatocyte growth factor receptor, c-Met control cancer cell growth, survival, and invasion. A large (6), and integrins (7). Major integrins expressed on breast epi- body of evidence suggests that the cooperation between in- thelial cells include α2β1, α3β1, αvβ3, αvβ5, αvβ6, α5β1, tegrin and receptor tyrosine kinase signaling regulates certain α6β1, and α6β4 (7). Among these, this review focuses on signaling functions that are important for cancer progression. αvβ3, α5β1, and α6β4, all of which are upregulated in in- Recent developments on the crosstalk between integrins and vasive breast carcinoma and have well established relation- receptor tyrosine kinases, and its implication in mammary tu- ships with RTKs (8). These integrins serve as receptors for vi- mor progression, are discussed. -
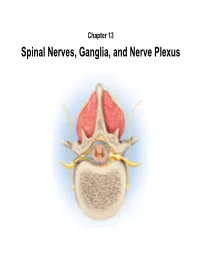
Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves
Chapter 13 Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves Posterior Spinous process of vertebra Posterior root Deep muscles of back Posterior ramus Spinal cord Transverse process of vertebra Posterior root ganglion Spinal nerve Anterior ramus Meningeal branch Communicating rami Anterior root Vertebral body Sympathetic ganglion Anterior General Anatomy of Nerves and Ganglia • Spinal cord communicates with the rest of the body by way of spinal nerves • nerve = a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue – mixed nerves contain both afferent (sensory) and efferent (motor) fibers – composed of thousands of fibers carrying currents in opposite directions Anatomy of a Nerve Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Epineurium Perineurium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endoneurium Nerve Rootlets fiber Posterior root Fascicle Posterior root ganglion Anterior Blood root vessels Spinal nerve (b) Copyright by R.G. Kessel and R.H. Kardon, Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy, 1979, W.H. Freeman, All rights reserved Blood vessels Fascicle Epineurium Perineurium Unmyelinated nerve fibers Myelinated nerve fibers (a) Endoneurium Myelin General Anatomy of Nerves and Ganglia • nerves of peripheral nervous system are ensheathed in Schwann cells – forms neurilemma and often a myelin sheath around the axon – external to neurilemma, each fiber is surrounded by -

Class I Histone Deacetylases in Retinal Progenitors and Differentiating Ganglion Cells
ACCEPTED MANUSCRIPT Class I Histone Deacetylases in Retinal Progenitors and Differentiating Ganglion Cells #Ankita Saha1, 2, #Sarika Tiwari1, 2, Subramanian Dharmarajan1, 2, *Deborah C. Otteson3, and *Teri L. Belecky-Adams1, 2 *Both of the authors are corresponding authors #These authors contributed equally to this work 1Department of Biology, Indiana University-Purdue University Indianapolis, 723 W Michigan St, Indianapolis, IN-46202. 2Center for Developmental and Regenerative Biology, Indiana University- Purdue University Indianapolis, 723 W Michigan St, Indianapolis, IN-46202. 3 University of Houston College of Optometry, 4901 Calhoun Rd. Rm 2195, Houston, TX 77204- 2020. Ankita Saha:[email protected] Sarika Tiwari: [email protected] Subramanian Dharmarajan: [email protected] MANUSCRIPT Keywords: Histone Deacetylases, HDACs, mouse, murine, retina, progenitors, retinal ganglion cells Running Title: HDAC Localization in Murine Retina Correspondence should be sent to the following addresses: Teri L Belecky-Adams Department of Biology, SL306 Center for DevelopmentalACCEPTED and Regenerative Biology Indiana University-Purdue University Indianapolis. 723 W Michigan St. Indianapolis, IN-46202 Tel. (317)278-5715 Fax (317) 274-2846 E-mail: [email protected] ___________________________________________________________________ This is the author's manuscript of the article published in final edited form as: Saha, A., Tiwari, S., Dharmarajan, S., Otteson, D. C., & Belecky-Adams, T. L. (2018). Class I histone deacetylases in retinal progenitors and differentiating ganglion cells. Gene Expression Patterns. https://doi.org/10.1016/j.gep.2018.08.007 ACCEPTED MANUSCRIPT Deborah C. Otteson University Eye Institute, Room 2195 University of Houston 4901 Calhoun Rd. Houston, TX 77204-2020 Tel. (713) 743-1952 Fax (713) 74302053 E-mail: [email protected] MANUSCRIPT ACCEPTED 2 ACCEPTED MANUSCRIPT Abstract Background: The acetylation state of histones has been used as an indicator of the developmental state of progenitor and differentiating cells. -

Notch-Signaling in Retinal Regeneration and Müller Glial Plasticity
Notch-Signaling in Retinal Regeneration and Müller glial Plasticity DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Kanika Ghai, MS Neuroscience Graduate Studies Program The Ohio State University 2009 Dissertation Committee: Dr. Andy J Fischer, Advisor Dr. Heithem El-Hodiri Dr. Susan Cole Dr. Paul Henion Copyright by Kanika Ghai 2009 ABSTRACT Eye diseases such as blindness, age-related macular degeneration (AMD), diabetic retinopathy and glaucoma are highly prevalent in the developed world, especially in a rapidly aging population. These sight-threatening diseases all involve the progressive loss of cells from the retina, the light-sensing neural tissue that lines the back of the eye. Thus, developing strategies to replace dying retinal cells or prolonging neuronal survival is essential to preserving sight. In this regard, cell-based therapies hold great potential as a treatment for retinal diseases. One strategy is to stimulate cells within the retina to produce new neurons. This dissertation elucidates the properties of the primary support cell in the chicken retina, known as the Müller glia, which have recently been shown to possess stem-cell like properties, with the potential to form new neurons in damaged retinas. However, the mechanisms that govern this stem-cell like ability are less well understood. In order to better understand these properties, we analyze the role of one of the key developmental processes, i.e., the Notch-Signaling Pathway in regulating proliferative, neuroprotective and regenerative properties of Müller glia and bestow them with this plasticity.