Primer: Tobacco Harm Reduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bridging the Gap: a Practitioner's Guide to Harm Reduction in Drug
Bridging the Gap A Practitioner’s Guide to Harm Reduction in Drug Courts by Alejandra Garcia and Dave Lucas a Author Alejandra Garcia, MSW Center for Court Innovation Dave Lucas, MSW Center for Court Innovation Acknowledgements Bridging the Gap: A Practitioners Guide to Harm Reduction in Drug Courts represents an ambitious reimagining of drug court practices through a harm reduction lens. It was born of two intersecting health emergencies—COVID-19 and the overdose crisis—and a belief that this moment calls for challenging conversations and bold change. Against this backdrop, Bridging the Gap’s first aim is plain: to elevate the safety, dignity, and autonomy of current and future drug court participants. It is also an invitation to practitioners to revisit and reflect upon drug court principles from a new vantage point. There are some who see the core tenets of drug courts and harm reduction as antithetical. As such, disagreement is to be expected. Bridging the Gap aspires to be the beginning of an evolving discussion, not the final word. This publication would not have been possible without the support of Aaron Arnold, Annie Schachar, Karen Otis, Najah Magloire, Matt Watkins, and Julian Adler. We are deeply grateful for your thoughtful advice, careful edits, and encouraging words. A special thanks also to our designers, Samiha Amin Meah and Isaac Gertman. Bridging the Gap is dedicated to anyone working to make the world a safer place for people who use drugs. Thanks to all who approach this document with an open mind. For more information, email [email protected]. -

Harm Reduction Interventions in Substance Abuse Treatment
Prepared by: Karissa Hughes April 2018 Harm Reduction Interventions in Substance Abuse Treatment 1 Philosophy/Overview ● Harm reduction is a client-centered philosophy that engages clients in the process of behavior change even if they are not motivated to pursue abstinence from substance 2 use or refrain from engaging in other risk-taking behaviors as a treatment goal. ● Harm reduction challenges the traditional notion of abstinence as a universal treatment goal for problem substance use. It focuses on reducing the harm of drug use to the user and society (health, social, and economic consequences) for people unable or unwilling to stop using drugs rather than requiring abstinence as a condition of treatment. o Harm reduction strategies are community-based, user-driven, non-judgmental and address systems that isolate and marginalize individuals. o Harm reduction acknowledges that clients often seek out substance abuse treatment services to address risky injection practices and sexual risk-taking behavior, even when they are not interested in changing substance use patterns. ● Many people who use drugs prefer to use informal and non-clinical methods to reduce their drug consumption or reduce the risks associated with their drug use. Thus, harm reduction is a public health philosophy and service delivery model to reduce the risks of drug use while respecting the dignity and autonomy of individuals. o Harm reduction policies and practices emphasize the human right for the highest attainable standard for health of people who use drugs. o The approach is based on the belief that it is in both the user's and society's best interest to minimize the adverse consequences of drug use when the person is unable or unwilling to discontinue using. -
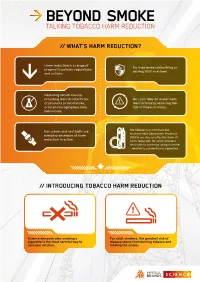
Talking Tobacco Harm Reduction
TALKING TOBACCO HARM REDUCTION // WHAT’S HARM REDUCTION? Harm reduction is a range of Do they make sunbathing or pragmatic policies, regulations driving 100% risk-free? and actions. Reducing health risks by providing less harmful forms No – but they do make them of products or substances, less harmful by reducing the or by encouraging less risky risk of illness or injury. behaviours. Sun cream and seat belts are We believe non-combustible nicotine Next Generation Products everyday examples of harm (NGPs) are also an effective form of reduction in action. harm reduction for adult smokers who wish to continue using nicotine – relative to conventional cigarettes. // INTRODUCING TOBACCO HARM REDUCTION Science demonstrates smoking a For adult smokers, the greatest risk of cigarette is the most harmful way to disease stems from burning tobacco and consume nicotine. inhaling the smoke. // WHAT IS THR? Tobacco smoke contains over 7000 The undisputed best action adult chemicals – nicotine is one of them. smokers can take to improve their Around 100 are classified by public health is to stop all tobacco and health experts as causes or potential nicotine use entirely, but many are not causes of smoking-related disease. interested or willing to take this step. Numerous public health bodies1 While the science suggests nicotine is believe transitioning to nicotine addictive and not risk-free, it’s neither products that are substantially less carcinogenic nor the primary cause of harmful than inhaled tobacco smoke smoking-related diseases. is their next best option – we agree. Contains 7000+ Contains chemicals, 100 significantly of them harmful fewer and lower or potentially levels of harmful harmful. -

Harm Reduction and Currently Illegal Drugs Implications for Nursing Policy, Practice, Education and Research
Harm Reduction and Currently Illegal Drugs Implications for Nursing Policy, Practice, Education and Research Discussion Paper This paper has been prepared by the Canadian Nurses Association (CNA) to stimulate dialogue on a particular topic or topics. The views and opinions expressed in this paper do not necessarily reflect the views of the CNA board of directors. CNA is the national professional voice of registered nurses in Canada. A federation of 11 provincial and territorial nursing associations and colleges representing 143,843 registered nurses, CNA advances the practice and profession of nursing to improve health outcomes and strengthen Canada’s publicly funded, not-for-profit health system. All rights reserved. No part of this document may be reproduced, stored in a retrieval system, or transcribed, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without written permission of the publisher. © Canadian Nurses Association 50 Driveway Ottawa, ON K2P 1E2 Tel.: 613-237-2133 or 1-800-361-8404 Fax: 613-237-3520 Website: www.cna-aiic.ca ISBN 978-1-55119-349-6 March 2011 TABLE OF CONTENTS Executive Summary ................................................................................................................. 3 Introduction ............................................................................................................................. 5 I. Illegal Drug Use in Canada .................................................................................................. 7 Health -

Comments to the FDA on Swedish Match
November 25, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA-2014-N-1051, Modified Risk Tobacco Product Applications: Applications for 10 Products Submitted by Swedish Match North America, Inc. The undersigned organizations submit these comments in response to the announcement for public comment of a modified risk tobacco product application by Swedish Match North America, Inc. (“Swedish Match” or “the company”) for 10 tobacco products.1 The Swedish Match application presents evidence to FDA that support the proposition that Swedish smokers who switch entirely to snus derive individual health benefits. Swedish Match also provides evidence indicating that the high prevalence of snus usage among men in Sweden has contributed to a lower frequency of tobacco-related disease and mortality than is found in comparable populations with higher cigarette smoking rates. This information suggests that if Swedish Match presents convincing evidence that being permitted to make a modified risk claim would lead smokers that would not otherwise quit smoking to switch completely to one of these Swedish snus products, without leading to significant use among youth, such an application would deserve serious consideration. However, as discussed at length in Part I below, the modified risk application submitted by Swedish Match in its current form must be denied by FDA because it is legally defective under federal law. Although Swedish Match seeks a modified risk order under Section 911 of the Food, Drug & Cosmetic Act (FDCA), as amended by the Family Smoking Prevention and Tobacco Control Act (“Tobacco Control Act” or “TCA”), Swedish Match does not propose to make a claim concerning the relative risk for the listed snus products. -

An Endgame for Tobacco? by the Various Proposals
Editorial Tob Control: first published as 10.1136/tobaccocontrol-2013-050989 on 15 April 2013. Downloaded from then to evaluate what might be achieved An endgame for tobacco? by the various proposals. The latter requires contemplation of each strategy’s Kenneth E Warner potential outcomes, good and undesirable, and the political, legal, ethical, economic, regulatory and social barriers to realising ABSTRACT great successes of many national anti- the strategy’s implementation. Finally, the Since its origins in the 1960s, tobacco control smoking campaigns notwithstanding, the very idea of what constitutes the end of has achieved remarkable success against the pandemic persists. In many developed the endgame needs to be addressed. scourge of tobacco-produced disease and countries with the most robust tobacco Strikingly, to date, there has never been a death. Yet tobacco use, especially cigarette control efforts, the decline in smoking serious discussion within the tobacco smoking, remains the world's leading cause of prevalence has slowed to a trickle. control community about what would preventable premature death and is likely to Evidence-based policies seem incapable of constitute a final victory in tobacco do so for decades to come. Evidence-based substantially hastening the demise of control. Would it be a reduction in 5 policies seem incapable of substantially smoking in these countries. In LMICs, smoking-produced deaths to, say, 10% of hastening the demise of smoking. Slowness in smoking is on the rise. The net effect is current levels? -

2.3 Harm Reduction – What Is It and Why Is It Useful?
SENSIBLE CANNABIS EDUCATION A Toolkit for Educating Youth 2.3 HARM REDUCTION – WHAT IS IT AND WHY IS IT USEFUL? By the end of this section, you will: 1. Understand what harm reduction is 2. Understand practical ways to reduce the harms associated with cannabis use, through both abstinence and the reduction of risky behaviours for youth who are already using cannabis WHAT IS HARM REDUCTION? “Taking a pragmatic approach to this generally understood phenomenon, harm reduction avoids taking a uniform stance that substance use is bad, but instead focuses on getting accurate and unbiased information on the harm of use to potential users, in order to help them make informed decisions about whether to use, and if they choose to use, what precautions to take to minimize their risk.”277 Harm reduction is a philosophy that underpins public health approaches to drugs and drug use, and attempts to reduce the harms of drug use without necessarily reducing drug use itself. Harm reduction acknowledges that there are inherent risks involved with a range of behaviours and that there are ways to reduce those risks. Harm reduction can also be understood in the context of a range of activities other than drug use, as simple as wearing sunscreen or wearing a helmet. REDUCING CANNABIS-RELATED HARMS In order to ensure cannabis education is suitable for all young people, discussing strategies to reduce the harms of cannabis use is of critical importance to supporting responsible and safe use among those youth who may choose to use cannabis. In 2017, the Canadian Research Initiative in Substance Misuse (CRISM) released an evidence- based guide on how to improve health and minimize risk for Canadians who use cannabis.278 The following discussion relies on CRISM’s “Lower-Risk Cannabis Use Guidelines” (LRCUG), however, it is tailored to youth based on feedback from our content committee and contributors. -

Reducing Cannabis Harms: a Guide for Ontario Campuses
Reducing cannabis harms: A guide for Ontario campuses About This Guide This guide explores issues related to cannabis use and provides readers with an overview of health approaches that can reduce the harms and risks associated with it. Any campus professional — whether faculty, academic advisor, counsellor, or student services professional — working with students who use cannabis will be able to refer to the guide for information. The first section will give you a better understanding of cannabis, the Ontario context, the substance use spectrum, as well as substance use disorders and problematic substance use. Section 2 looks at the reasons why students use or don’t use cannabis, the effects of cannabis use on the brain in adolescents and young adults, the link between cannabis use and mental health, the effects of language and stigma, and strategies that campus professionals can use to reduce harms when directly engaging with students. The final section provides concrete steps for developing a campus-wide cannabis-use framework to reduce harm. While the focus of this guide is on cannabis use by students, not campus staff or faculty, this is in no way meant to minimize the need to address the broader scope of mental health, substance use, and well- being on campuses, including among faculty and staff. Each post-secondary institution has unique strengths, circumstances, and needs. For this reason, while the broad areas addressed in this guide are relevant to all institutions, it is not meant to be prescriptive or to serve as legal advice pertaining to cannabis use legislation. As of its writing, the information in this guide is accurate and reflects current research and legislation. -

Harm Reduction / Drug Policy Reform Notes
Questions and answers on the issue of a regulated market for currently illegal drugs Mark Haden Vancouver Coastal Health Draft: February 2012 For the past decade I have been involved with public presentations which explore the failures of drug prohibition. In these presentations I recommend a public health, human rights model of drug control which brings all currently illegal drugs into a regulatory framework. The following is a list of the most common challenges I experience: Q1: Are you saying that you want to legalize all drugs? A: No: Legalization is often interpreted to mean that the current capitalist free market system would be used to advertise and distribute currently illegal drugs. The goal of the free market is to increase consumption. The concept of a regulated market is instead guided by Public Health which has the goal to reduce the health and social problems associated with drugs. Q2: What do you mean by a regulated market for illegal drugs? A: A regulated market would actively control drugs based on the principles of public health and human rights. Prohibition paradoxically stimulates a illegal market that makes concentrated and sometimes toxic, drugs widely available. The goal is to greatly reduce or shut down the illegal market and regulate drugs in a way that reduces harm to individuals, families and our society as a whole. Seeing drug use as primarily a health and social issue rather than a criminal issue allows us to explore a wide range of tools to manage the problems associated with drugs in a more effective way. Q3: Are you saying “yes” to drugs? A: No – but we are saying “yes” to using more effective ways of controlling drugs and their problems. -

A FOCUS on HARM REDUCTION Why It
A FOCUS ON HARM REDUCTION Why it mattersSUSTAINABILITY FOCUS REPORT 2013: How we address the public health impact of our products A nicotine molecule In this report Focusing on the facts 2 At the core of our business strategy 3 Innovative nicotine products 4 ‘Safer’ tobacco products: the research 7 So, what’s next? 9 Our Chief Executive on why it matters b British American Tobacco Harm reduction: sustainability focus report 2013 Because it’s crucial to the future of our business. Surely tobacco harm reduction should just be about What areas are you concentrating on? getting people to quit smoking? Our approach to harm reduction has two distinct areas: The only way to be certain of avoiding the serious health risks nicotine-based alternatives and reduced-risk tobacco products. associated with smoking is not to smoke at all. However, despite In the nicotine category, we have established a stand-alone increasingly strict tobacco control policies, many people continue business solely dedicated to this area. This brings together our to smoke. And the World Health Organisation estimates that existing Nicoventures business with CN Creative, the e-cigarette 1 many more will do so in the future . So realistically the ‘quit or die’ company we acquired at the end of last year, into a single business approach to reducing the public health impact of smoking simply which will continue to operate under the Nicoventures name. isn’t enough. This business has already launched its first e-cigarette in the UK, For adults that choose to continue to smoke, tobacco harm which will be expanded into further markets in the coming year. -

List of References Submitted During the Public Consultation
Bibliography of the Sources Examined for the Opinion on the Addictiveness and Attractiveness of Tobacco Additives ACSCAN (American Cancer Society Cancer Action Network), American Heart Association, American Lung Association, Campaign for Tobacco-Free Kids, American Legacy Foundation. Submission to U.S. Food and Drug Administration re Docket No. FDA- 2010-N-0207, 2010; July 26. Available from: URL: http://www.tobaccofreekids.org/reports/fda/regulatory_docs/Comments%20to%20 FDA%20RFI%20re%20youth%20and%20minority%20marketing%207-26-10.pdf Adam T, McAughey J, McGrath C, Mocker C, Zimmermann R. Simultaneous on-line size and chemical analysis of gas phase and particulate phase of cigarette mainstream smoke. Anal Bioanal Chem 2009; 394:1193-203. Adam T, Mitschke S, Streibel T, Baker RR, Zimmermann R. Puff-by-puff resolved characterisation of cigarette mainstream smoke by single photon ionisation (SPI)- time-of-flight mass spectrometry (TOFMS): comparison of the 2R4F research cigarette and pure Burley, Virginia, Oriental and Maryland tobacco cigarettes. Anal Chim Acta. 2006 Jul 21;572(2):219-29. Epub 2006; May 22. Ahijevych K, Garrett BE. Menthol pharmacology and its potential impact on cigarette smoking behaviour. Nicotine Tob Res 2004; 6 Suppl 1:S17-28. Ahijevych K, Gillespie J, Demirci M, Jagadeesh J. Menthol and nonmenthol cigarettes and smoke exposure in black and white women. Pharmacol Biochem Behav 1996; 53:355-60. Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav 1999; 24:115-20. Akehurst BC Tobacco. Second edition. Tropical Agriculture Series. Longman. London and New York. 1981; Pp. -

Tobacco Control
TOBACCO CONTROL Playbook World Health Organization ABSTRACT Tobacco control is difficult and complex and obstructed by the tactics of the tobacco industry and its allies to oppose effective tobacco control measures. This document was developed by the WHO Regional Office for Europe by collecting numerous evidence-based arguments from different thematic areas, reflecting the challenges that tobacco control leaders have faced while implementing various articles of the WHO FCTC and highlighting arguments they have developed in order to counter and succeed against the tobacco industry. KEY WORDS TOBACCO CONTROL WHO FCTC HEALTH EFFECTS TOBACCO INDUSTRY ARGUMENTS © World Health Organization 2019 All rights reserved. The Regional Office for Europe of the World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication.