Varicella-Zoster Virus Epstein Barr Virus, and Human Herpesviruses (Hhv) 6, 7, and 8
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NIH Medlineplus Magazine Winter 2010
Trusted Health Information from the National Institutes of Health ® NIHMedlineWINTER 2010 Plusthe magazine Plus, in this issue! • Treating “ Keep diverticulitis the beat” Healthy blood Pressure • Protecting Helps Prevent Heart disease Yourself from Shingles • Progress against Prostate cancer • Preventing Suicide in Young Adults • relieving the Model Heidi Klum joins The Heart Truth Pain of tMJ Campaign for women’s heart health. • The Real Benefits of Personalized Prevent Heart Medicine Disease Now! You can lower your risk. A publication of the NatioNal Institutes of HealtH and the frieNds of the NatioNal library of MediciNe FRIENDS OF THE NATIONAL LIBRARY OF MEDICINE Saying “Yes!” to Careers in Health Care ecently, the Friends of NLM was delighted to co-sponsor the fourth annual “Yes, I Can Be a Healthcare Professional” conference at Frederick Douglass Academy in Harlem. More than 2,300 students and parents from socioeconomically disadvantaged communities throughout the entire New York City metropolitan area convened for Rthe daylong session. It featured practical skills workshops, discussion groups, and exhibits from local educational institutions, health professional societies, community health services, and health information providers, including the National Library of Medicine (NLM). If you’ll pardon the expression, the enthusiasm among the attendees—current and future Photo: NLM Photo: healthcare professionals—was infectious! donald West King, M.d. fNlM chairman It was especially exciting to mix with some of the students from six public and charter high schools in Harlem and the South Bronx enrolled in the Science and Health Career Exploration Program. The program was created by Mentoring in Medicine, Inc., funded by the NLM and Let Us Hear co-sponsored by the Friends. -

Human Herpesvirus 6 in Cerebrospinal Fluid of Patients Infected with HIV
J Neurol Neurosurg Psychiatry 1999;67:789–792 789 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.67.6.789 on 1 December 1999. Downloaded from SHORT REPORT Human herpesvirus 6 in cerebrospinal fluid of patients infected with HIV: frequency and clinical significance Simona Bossolasco, Roberta Marenzi, Helena Dahl, Luca Vago, Maria Rosa Terreni, Francesco Broccolo, Adriano Lazzarin, Annika Linde, Paola Cinque Abstract convulsions or other neurological symptoms The objective was to evaluate the fre- complicating exanthema subitum.2–4 HHV-6 quency of human herpesvirus 6 (HHV-6) encephalitis or milder neurological symptoms DNA detection in the CSF of patients have been described in both immunocompet- infected with HIV and its relation to brain ent adults and transplanted patients,5–7 and the disease and systemic HHV-6 infection. virus might also play an aetiological part in Nested polymerase chain reaction multiple sclerosis.8 HHV-6 has also been found (PCR) was used to analyse CSF samples in the brain or CSF of adults and children with from 365 consecutive HIV infected pa- AIDS, but its role in neurological disease is still tients with neurological symptoms. When unclear.9–12 available, plasma and brain tissues from To study the possible relation between patients whose CSF was HHV-6 positive HHV-6 and brain disease in patients infected were also studied. with HIV, the presence of HHV-6-DNA in the HHV-6 was found in the CSF of eight of CSF was assessed in a large group of patients copyright. Division of Infectious the 365 patients (2.2%): two had type A with neurological disease and was correlated Diseases, San RaVaele and four type B; the HHV-6 variant could with clinical and neuropathology patterns. -

HIV Infection and AIDS
G Maartens 12 HIV infection and AIDS Clinical examination in HIV disease 306 Prevention of opportunistic infections 323 Epidemiology 308 Preventing exposure 323 Global and regional epidemics 308 Chemoprophylaxis 323 Modes of transmission 308 Immunisation 324 Virology and immunology 309 Antiretroviral therapy 324 ART complications 325 Diagnosis and investigations 310 ART in special situations 326 Diagnosing HIV infection 310 Prevention of HIV 327 Viral load and CD4 counts 311 Clinical manifestations of HIV 311 Presenting problems in HIV infection 312 Lymphadenopathy 313 Weight loss 313 Fever 313 Mucocutaneous disease 314 Gastrointestinal disease 316 Hepatobiliary disease 317 Respiratory disease 318 Nervous system and eye disease 319 Rheumatological disease 321 Haematological abnormalities 322 Renal disease 322 Cardiac disease 322 HIV-related cancers 322 306 • HIV INFECTION AND AIDS Clinical examination in HIV disease 2 Oropharynx 34Neck Eyes Mucous membranes Lymph node enlargement Retina Tuberculosis Toxoplasmosis Lymphoma HIV retinopathy Kaposi’s sarcoma Progressive outer retinal Persistent generalised necrosis lymphadenopathy Parotidomegaly Oropharyngeal candidiasis Cytomegalovirus retinitis Cervical lymphadenopathy 3 Oral hairy leucoplakia 5 Central nervous system Herpes simplex Higher mental function Aphthous ulcers 4 HIV dementia Kaposi’s sarcoma Progressive multifocal leucoencephalopathy Teeth Focal signs 5 Toxoplasmosis Primary CNS lymphoma Neck stiffness Cryptococcal meningitis 2 Tuberculous meningitis Pneumococcal meningitis 6 -

Varicella (Chickenpox): Questions and Answers Q&A Information About the Disease and Vaccines
Varicella (Chickenpox): Questions and Answers Q&A information about the disease and vaccines What causes chickenpox? more common in infants, adults, and people with Chickenpox is caused by a virus, the varicella-zoster weakened immune systems. virus. How do I know if my child has chickenpox? How does chickenpox spread? Usually chickenpox can be diagnosed by disease his- Chickenpox spreads from person to person by direct tory and appearance alone. Adults who need to contact or through the air by coughing or sneezing. know if they’ve had chickenpox in the past can have It is highly contagious. It can also be spread through this determined by a laboratory test. Chickenpox is direct contact with the fluid from a blister of a per- much less common now than it was before a vaccine son infected with chickenpox, or from direct contact became available, so parents, doctors, and nurses with a sore from a person with shingles. are less familiar with it. It may be necessary to perform laboratory testing for children to confirm chickenpox. How long does it take to show signs of chickenpox after being exposed? How long is a person with chickenpox contagious? It takes from 10 to 21 days to develop symptoms after Patients with chickenpox are contagious for 1–2 days being exposed to a person infected with chickenpox. before the rash appears and continue to be conta- The usual time period is 14–16 days. gious through the first 4–5 days or until all the blisters are crusted over. What are the symptoms of chickenpox? Is there a treatment for chickenpox? The most common symptoms of chickenpox are rash, fever, coughing, fussiness, headache, and loss of appe- Most cases of chickenpox in otherwise healthy children tite. -

Hairy Leukoplakia James E
Marquette University e-Publications@Marquette School of Dentistry Faculty Research and Dentistry, School of Publications 5-5-2017 Hairy Leukoplakia James E. Cade Meharry Medical College School of Dentistry Richard P. Vinson Paul L Foster School of Medicine Jeff urB gess University of Washington School of Dental Medicine Sanjiv S. Agarwala Temple University Shool of Medicine Denis P. Lynch Marquette University, [email protected] See next page for additional authors Published version. Medscape Drugs & Diseases (May 5, 2017). Publisher link. © 2017 by WebMD LLC. Used with permission. Authors James E. Cade, Richard P. Vinson, Jeff urB gess, Sanjiv S. Agarwala, Denis P. Lynch, and Gary L. Stafford This blog post/website is available at e-Publications@Marquette: https://epublications.marquette.edu/dentistry_fac/252 Overview Background Oral hairy leukoplakia (OHL) is a disease of the mucosa first described in 1984. This pathology is associated with Epstein-Barr virus (EBV) and occurs mostly in people with HIV infection, both immunocompromised and immunocompetent, and can affect patients who are HIV negative.{ref1}{ref2} The first case in an HIV-negative patient was reported in 1999 in a 56-year-old patient with acute lymphocytic leukemia. Later, many cases were reported in heart, kidney, and bone marrow transplant recipients and patients with hematological malignancies.{ref3}{ref4} Pathophysiology The Epstein-Barr virus (EBV), a ubiquitous herpesvirus estimated to infect 90% of the world's population, is linked to a growing number of diseases, especially in immunocompromised hosts. Like all herpesviruses, EBV establishes a life-long, persistent infection of its host. The pathogenesis of hairy leukoplakia is clearly complex, potentially requiring a convergence of factors including EBV co-infection, productive EBV replication, EBV genetic evolution, expression of specific EBV "latent" genes, and immune escape. -
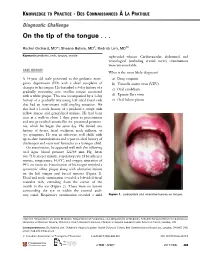
On the Tip of the Tongue
KNOWLEDGE TO PRACTICE DES CONNAISSANCES ÀLA PRATIQUE Diagnostic Challenge On the tip of the tongue . Rachel Orchard, MD*; Sheena Belisle, MD†; Rodrick Lim, MD†‡ Keywords: pediatric, rash, tongue, vesicle right-sided wheeze. Cardiovascular, abdominal, and neurological (including cranial nerve) examinations were unremarkable. CASE HISTORY What is the most likely diagnosis? A 14-year-old male presented to the pediatric emer- a) Drug eruption gency department (ED) with a chief complaint of b) Varicella zoster virus (VZV) changes to his tongue. He described a 3-day history of a c) Oral candidiasis gradually worsening sore, swollen tongue associated with a white plaque. This was accompanied by a 3-day d) Epstein-Barr virus history of a gradually worsening left-sided facial rash e) Oral lichen planus that had an intermittent mild tingling sensation. He also had a 1-week history of a productive cough with yellow mucus and generalized malaise. He had been seen at a walk-in clinic 2 days prior to presentation and was prescribed amoxicillin for presumed pneumo- nia, which he began the same day. He denied any history of fevers, facial weakness, neck stiffness, or eye symptoms. He was an otherwise well child, with up-to-date immunizations and a past medical history of chickenpox and recurrent furuncles as a younger child. On examination, he appeared well with the following vital signs: blood pressure 122/64 mm Hg, heart rate 73 beats per minute, respiratory rate 18 breaths per minute, temperature 36.8°C, and oxygen saturation of 99% on room air. Examination of his tongue revealed a symmetric white plaque along with ulcerative lesions on the left tongue and buccal mucosa (Figure 1). -

Herpes Zoster (Shingles
HHERPES ZZOSTER ((SSHINGLES)) www.theeyecenter.com Definition This infection is produced by the varicella-zoster virus, the same virus that causes chickenpox. After an initial outbreak of chickenpox (often during childhood), the virus remains inactive within the nerve cells of the central nervous system. But in some people, the varicella-zoster virus will reactivate at another time in their lives. When this occurs, the virus travels down long nerve fibers and infects some part of the body, producing a blistering rash (shingles), fever, painful inflammations of the affected nerve fibers, and a general feeling of sluggishness. Symptoms Varicella-zoster virus may travel to the head and neck, perhaps involving an eye, part of the nose, cheek, and forehead. In about 40 percent of those with shingles in these areas, the virus infects the cornea. Doctors will often prescribe oral anti-viral treatment to reduce the risk of the virus-infecting cells deep within the tissue, which could inflame and scar the cornea. The disease may also cause decreased corneal sensitivity, meaning that foreign matter, such as eyelashes, in the eye are not felt as intensely. For many, this decreased sensitivity will be permanent. Alexandria Fairfax Sterling Leesburg 703-931-9100 703-573-8080 703-430-4400 703-858-3170 Typical symptoms are as follows: • Pain • Inflammation (swelling and puffiness) • Redness • Sensitivity to light • Blurred vision If the herpes zoster infections continue, long-term affects could include: • Permanent scarring of the cornea, retina, optic nerve or conjunctiva • Glaucoma • Cataracts At-Risk Groups Although shingles can occur in anyone exposed to the varicella-zoster virus, research has established two general risk factors for the disease: (1) Advanced age; and (2) A weakened immune system. -

Oral Leukoplakia
Division of Oral Medicine and Dentistry Oral Leukoplakia What is oral leukoplakia? What causes oral leukoplakia? Oral leukoplakia (leuko=white, plakia=patch) is a white patch in Alcohol and tobacco use, both known risk factors for the mouth that cannot be rubbed of and cannot be diagnosed oral cancer, are similarly well-established risk factors for as any other condition. Lichen planus, yeast infections development of oral leukoplakia. Other risk factors include a (“thrush”), chronic cheek and tongue chewing injuries, and weakened immune system, long-term treatment with immune hairy/coated tongue are some of the specifc conditions suppressing medications, a personal or family history of cancer, that appear white in the mouth and are therefore NOT oral and, in some cultures, the chewing of areca nut and betel leaf. In leukoplakia. When all such known conditions have been ruled many patients with oral leukoplakia, however, there are no risk out, a patient is diagnosed with oral leukoplakia. While the factors and we don’t know why it develops. long-term history of these lesions is impossible to predict, it How do we know it is oral leukoplakia? is known that true leukoplakias are considered “potentially malignant,” meaning that they have the potential, over time, to If your doctor suspects that a white lesion in your mouth is due develop into oral cancer. to irritation, the source of the irritation will be removed and you will be asked to return in a few weeks for re-evaluation. If the Oral leukoplakia occurs in 1-2% of the population and is most white area is still present at the next visit, a biopsy will likely be common in patients over age 40. -

1. Oral Infections.Pdf
ORAL INFECTIONS Viral infections Herpes Human Papilloma Viruses Coxsackie Paramyxoviruses Retroviruses: HIV Bacterial Infections Dental caries Periodontal disease Pharyngitis and tonsillitis Scarlet fever Tuberculosis - Mycobacterium Syphilis -Treponema pallidum Actinomycosis – Actinomyces Gonorrhea – Neisseria gonorrheae Osteomyelitis - Staphylococcus Fungal infections (Mycoses) Candida albicans Histoplasma capsulatum Coccidioides Blastomyces dermatitidis Aspergillus Zygomyces CDE (Oral Pathology and Oral Medicine) 1 ORAL INFECTIONS VIRAL INFECTIONS • Viruses consist of: • Single or double strand DNA or RNA • Protein coat (capsid) • Often with an Envelope. • Obligate intracellular parasites – enters host cell in order to replicate. • 3 most commonly encountered virus families in the oral cavity: • Herpes virus • Papovavirus (HPV) • Coxsackie virus (an Enterovirus). DNA Viruses: A. HUMAN HERPES VIRUS (HHV) GROUP: 1. HERPES SIMPLEX VIRUS • Double stranded DNA virus. • 2 types: HSV-1 and HSV-2. • Lytic to human epithelial cells and latent in neural tissue. Clinical features: • May penetrate intact mucous membrane, but requires breaks in skin. • Infects peripheral nerve, migrates to regional ganglion. • Primary infection, latency and recurrence occur. • 99% of cases are sub-clinical in childhood. • Primary herpes: Acute herpetic gingivostomatitis. • 1% of cases; severe symptoms. • Children 1 - 3 years; may occur in adults. • Incubation period 3 – 8 days. • Numerous small vesicles in various sites in mouth; vesicles rupture to form multiple small shallow punctate ulcers with red halo. • Child is ill with fever, general malaise, myalgia, headache, regional lymphadenopathy, excessive salivation, halitosis. • Self limiting; heals in 2 weeks. • Immunocompromised patients may develop a prolonged form. • Secondary herpes: Recurrent oral herpes simplex. • Presents as: a) herpes labialis (cold sores) or b) recurrent intra-oral herpes – palate or gingiva. -

Oral Diseases Associated with Human Herpes Viruses: Aetiology, Clinical Features, Diagnosis and Management
www.sada.co.za / SADJ Vol 71 No. 6 CLINICAL REVIEW < 253 Oral diseases associated with human herpes viruses: aetiology, clinical features, diagnosis and management SADJ July 2016, Vol 71 no 6 p253 - p259 R Ballyram1, NH Wood2, RAG Khammissa3, J Lemmer4, L Feller5 ABSTRACT Human herpesviruses (HHVs) are very prevalent DNA ACRONYMS viruses that can cause a variety of orofacial diseases. EM: erythema multiforme Typically they are highly infectious, are contracted early in HHV: human herpes virus life, and following primary infection, usually persist in a latent form. Primary oral infections are often subclinical, but may PCR: polymerase chain reaction be symptomatic as in the case of herpes simplex virus- HSV, HHV-1: herpes simplex virus induced primary herpetic gingivostomatitis. Reactivation VZV, HHV-3: varicella-zoster virus of the latent forms may result in various conditions: herpes EBV, HHV-4: Epstein-Barr virus simplex virus (HSV) can cause recurrent herpetic orolabial CMV, HHV-5: cytomegalovirus lesions; varicella zoster virus (VZV) can cause herpes zoster; Epstein-Barr virus (EBV) can cause oral hairy Key words: herpes simplex virus, human herpes virus-8, leukoplakia; and reactivation of HHV-8 can cause Kaposi varicella zoster virus, Epstein-Barr virus, recurrent herpes sarcoma. In immunocompromised subjects, infections labialis, recurrent intraoral herpetic ulcers, treatment, val- with human herpesviruses are more extensive and aciclovir, aciclovir, famcicylovir. severe than in immunocompetent subjects. HSV and VZV infections are treated with nucleoside analogues aciclovir, valaciclovir, famciclovir and penciclovir. These agents INTRODucTION have few side effects and are effective when started The human herpesvirus (HHV) family comprises a diverse early in the course of the disease. -

Herpesviral Latency—Common Themes
pathogens Review Herpesviral Latency—Common Themes Magdalena Weidner-Glunde * , Ewa Kruminis-Kaszkiel and Mamata Savanagouder Department of Reproductive Immunology and Pathology, Institute of Animal Reproduction and Food Research of Polish Academy of Sciences, Tuwima Str. 10, 10-748 Olsztyn, Poland; [email protected] (E.K.-K.); [email protected] (M.S.) * Correspondence: [email protected] Received: 22 January 2020; Accepted: 14 February 2020; Published: 15 February 2020 Abstract: Latency establishment is the hallmark feature of herpesviruses, a group of viruses, of which nine are known to infect humans. They have co-evolved alongside their hosts, and mastered manipulation of cellular pathways and tweaking various processes to their advantage. As a result, they are very well adapted to persistence. The members of the three subfamilies belonging to the family Herpesviridae differ with regard to cell tropism, target cells for the latent reservoir, and characteristics of the infection. The mechanisms governing the latent state also seem quite different. Our knowledge about latency is most complete for the gammaherpesviruses due to previously missing adequate latency models for the alpha and beta-herpesviruses. Nevertheless, with advances in cell biology and the availability of appropriate cell-culture and animal models, the common features of the latency in the different subfamilies began to emerge. Three criteria have been set forth to define latency and differentiate it from persistent or abortive infection: 1) persistence of the viral genome, 2) limited viral gene expression with no viral particle production, and 3) the ability to reactivate to a lytic cycle. This review discusses these criteria for each of the subfamilies and highlights the common strategies adopted by herpesviruses to establish latency. -

Absence of Langerhans Cells in Oral Hairy Leukoplakia, an AIDS-Associated Lesion
Absence of Langerhans Cells in Oral Hairy Leukoplakia, an AIDS-Associated Lesion Troy E. D aniels, D .D .S., M .S. , Deborah Greenspan, B.D.S. , John S. Greenspan, B. D .S., Ph.D ., Evelyne Lennette, Ph.D., Morten Schi0dt, D.D.S. , D r. Odont. , Vibeke Peters en, B. S., and Yvonne de Souza, M .S. Department of Stomatology, School of Dentistry, University of Ca li fo rnia (TED, DG, JSG , MS, VP, YdeS), Sa n Francisco, California; and Virolab In c. (EL), Berkeley, Cali fo rnia, U .S.A. O ral hairy leuko plakia (HL) is a recently described mani tien ts, LC were detected w ith all 3 antibodies in 11/12 festati on o f human immunode fi ciency virus (HlV) infec specimens (92%) and were found in approx imately norm al tion in w hich Epstein-Barr virus (EB V) has been sh own numbers w ith at least 1 antibo d y. T here was cl ose corre to replicate. T o seek evidence fo r a local defect in mucosal lati on between the absen ce o f LC and positive staining for immunity, w e assessed the presence o f epithelial Lan ger E BV, human papillo m a virus antigen s, and candidal h yphae h ans cells (LC ) in these lesions and in autologous no nle in the epithelium. W e conclude that LC are absent or greatl y sional mucosa. W e used monoclonal antibodies against HLA reduced in the lesions o f HL. Absen ce of n o rmal LC func DR, HLA-DQ, and T 6 antigens to identify LC in bio psy ti on m ay be impo rtant in the pathogenesis o f HL and m ay specimens of HL fro m 23 ho m osexual m en.