Reduced Motor Neuron Excitability Is an Important Contributor to Weakness in a Rat Model of Sepsis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -

Are Astrocytes Executive Cells Within the Central Nervous System? ¿Son Los Astrocitos Células Ejecutivas Dentro Del Sistema Nervioso Central? Roberto E
DOI: 10.1590/0004-282X20160101 VIEW AND REVIEW Are astrocytes executive cells within the central nervous system? ¿Son los astrocitos células ejecutivas dentro del Sistema Nervioso Central? Roberto E. Sica1, Roberto Caccuri1, Cecilia Quarracino1, Francisco Capani1 ABSTRACT Experimental evidence suggests that astrocytes play a crucial role in the physiology of the central nervous system (CNS) by modulating synaptic activity and plasticity. Based on what is currently known we postulate that astrocytes are fundamental, along with neurons, for the information processing that takes place within the CNS. On the other hand, experimental findings and human observations signal that some of the primary degenerative diseases of the CNS, like frontotemporal dementia, Parkinson’s disease, Alzheimer’s dementia, Huntington’s dementia, primary cerebellar ataxias and amyotrophic lateral sclerosis, all of which affect the human species exclusively, may be due to astroglial dysfunction. This hypothesis is supported by observations that demonstrated that the killing of neurons by non-neural cells plays a major role in the pathogenesis of those diseases, at both their onset and their progression. Furthermore, recent findings suggest that astrocytes might be involved in the pathogenesis of some psychiatric disorders as well. Keywords: astrocytes; physiology; central nervous system; neurodegenerative diseases. RESUMEN Evidencias experimentales sugieren que los astrocitos desempeñan un rol crucial en la fisiología del sistema nervioso central (SNC) modulando la actividad y plasticidad sináptica. En base a lo actualmente conocido creemos que los astrocitos participan, en pie de igualdad con las neuronas, en los procesos de información del SNC. Además, observaciones experimentales y humanas encontraron que algunas de las enfermedades degenerativas primarias del SNC: la demencia fronto-temporal; las enfermedades de Parkinson, de Alzheimer, y de Huntington, las ataxias cerebelosas primarias y la esclerosis lateral amiotrófica, que afectan solo a los humanos, pueden deberse a astroglíopatía. -

11 Introduction to the Nervous System and Nervous Tissue
11 Introduction to the Nervous System and Nervous Tissue ou can’t turn on the television or radio, much less go online, without seeing some- 11.1 Overview of the Nervous thing to remind you of the nervous system. From advertisements for medications System 381 Yto treat depression and other psychiatric conditions to stories about celebrities and 11.2 Nervous Tissue 384 their battles with illegal drugs, information about the nervous system is everywhere in 11.3 Electrophysiology our popular culture. And there is good reason for this—the nervous system controls our of Neurons 393 perception and experience of the world. In addition, it directs voluntary movement, and 11.4 Neuronal Synapses 406 is the seat of our consciousness, personality, and learning and memory. Along with the 11.5 Neurotransmitters 413 endocrine system, the nervous system regulates many aspects of homeostasis, including 11.6 Functional Groups respiratory rate, blood pressure, body temperature, the sleep/wake cycle, and blood pH. of Neurons 417 In this chapter we introduce the multitasking nervous system and its basic functions and divisions. We then examine the structure and physiology of the main tissue of the nervous system: nervous tissue. As you read, notice that many of the same principles you discovered in the muscle tissue chapter (see Chapter 10) apply here as well. MODULE 11.1 Overview of the Nervous System Learning Outcomes 1. Describe the major functions of the nervous system. 2. Describe the structures and basic functions of each organ of the central and peripheral nervous systems. 3. Explain the major differences between the two functional divisions of the peripheral nervous system. -

Astrocytes Are the Primary Source of Tissue Factor in the Murine Central Nervous System
Astrocytes are the primary source of tissue factor in the murine central nervous system. A role for astrocytes in cerebral hemostasis. M Eddleston, … , T S Edgington, N Mackman J Clin Invest. 1993;92(1):349-358. https://doi.org/10.1172/JCI116573. Research Article Hemostasis in the brain is of paramount importance because bleeding into the neural parenchyma can result in paralysis, coma, and death. Consistent with this sensitivity to hemorrhage, the brain contains large amounts of tissue factor (TF), the major cellular initiator of the coagulation protease cascades. However, to date, the cellular source for TF in the central nervous system has not been identified. In this study, analysis of murine brain sections by in situ hybridization demonstrated high levels of TF mRNA in cells that expressed glial fibrillary acidic protein, a specific marker for astrocytes. Furthermore, primary mouse astrocyte cultures and astrocyte cell lines from mouse, rat, and human constitutively expressed TF mRNA and functional protein. These data indicated that astrocytes are the primary source of TF in the central nervous system. We propose that astrocytes forming the glia limitans around the neural vasculature and deep to the meninges are intimately involved in controlling hemorrhage in the brain. Finally, we observed an increase in TF mRNA expression in the brains of scrapie-infected mice. This modulation of TF expression in the absence of hemorrhage suggested that TF may function in processes other than hemostasis by altering protease generation in normal and diseased brain. Find the latest version: https://jci.me/116573/pdf Astrocytes Are the Primary Source of Tissue Factor in the Murine Central Nervous System A Role for Astrocytes in Cerebral Hemostasis Michael Eddleston, * Juan Carlos de la Torre,* Michael B. -

THE Mandffiular GANGLION - a NEW PERIPHERAL GANGLION of the LOCUST
J. exp. Biol. 148, 313-324 (1990) 313 Primed in Great Britain © The Company of Biologists Limited 1990 THE MANDffiULAR GANGLION - A NEW PERIPHERAL GANGLION OF THE LOCUST BY PETER BRAUNIG Institut fiir Zoologie, Technische Universitdt Milnchen, Lichtenbergstrafle 4, D-8046 Garching, Federal Republic of Germany Accepted 22 August 1989 Summary Paired peripheral ganglia within the locust mandibular segment are described. Each mandibular ganglion contains the cell bodies of 22-25 neurones. Four of these are sensory neurones which innervate the receptor strand of one of the mandibular proprioceptors. The other neurones connect the suboesophageal ganglion with the tritocerebral lobes of the brain, and with the first ganglion of the stomatogastric nervous system, the frontal ganglion. Introduction In addition to the chain of segmental ganglia of the central nervous system (CNS), insects possess a stomatogastric nervous system which innervates the foregut. It consists of the unpaired frontal and hypocerebral ganglia and the paired paraventricular ganglia. It is connected with the central nervous system (CNS) via the frontal connectives, which link the frontal ganglion to both tritocerebral lobes of the supraoesophageal ganglion, or brain (see Fig. 1). Nerve cells making connections with the frontal ganglion have been found chiefly in the brain, but there are also a few in the suboesophageal ganglion (Aubele and Klemm, 1977; Gundel and Penzlin, 1978; Kirby etal. 1984). Thus, both head ganglia appear to participate in the control of the stomatogastric nervous system. In the course of a study of the peripheral nervous system of the locust suboesophageal ganglion (Braunig, 1987), peculiar structures were found in association with one of the major branches of the mandibular nerve. -

Astrocyte Failure As a Cause of CNS Dysfunction
Molecular Psychiatry (2000) 5, 230–232 2000 Macmillan Publishers Ltd All rights reserved 1359-4184/00 $15.00 www.nature.com/mp NEWS & VIEWS Astrocyte failure as a cause of CNS dysfunction All insults to the central nervous systems (CNS), expressing HSV-Tk from the mouse Gfap promoter, including injury, ischemia, infection and degenerative reactive, transgene-expressing astrocytes adjacent to a disease are invariably accompanied by the hypertro- forebrain stab injury are ablated by GCV.8,9 These and phy, altered gene expression and proliferation of astro- other studies have demonstrated the essential nature of cytes, a process commonly referred to as ‘reactive astrocyte functions in a number of contexts related to astrocytosis’. While much is known about molecules the response to injury, and highlighted how astrocyte that either influence, or are produced by, reactive astro- failure might lead to CNS dysfunction in various ways. cytes,1,2 the functions of these cells are incompletely understood. Astrocytes are the most numerous cells in Astrocytes, the blood–brain barrier and interstitial the vertebrate central nervous system (CNS), and vari- edema ous functions have been ascribed to them in the unin- jured CNS, including: provision of structural support The anatomical correlate of the BBB is thought to for neural elements (neuro-glia = neural ‘glue’); homeo- reside in tight junctions between endothelial cells of static maintenance of the extracellular ionic environ- cerebral capillaries, which are of high electrical resist- ment and pH; -

Glia As a Link Between Neuroinflammation and Neuropathic Pain
http://dx.doi.org/10.4110/in.2012.12.2.41 REVIEW ARTICLE pISSN 1598-2629 eISSN 2092-6685 Glia as a Link between Neuroinflammation and Neuropathic Pain Mithilesh Kumar Jha, Sangmin Jeon and Kyoungho Suk* Department of Pharmacology, Brain Science & Engineering Institute, Kyungpook National University School of Medicine, Daegu 700-422, Korea Contemporary studies illustrate that peripheral injuries acti- clude astrocytes and oligodendrocytes (2). Glial cells that vate glial components of the peripheral and central cellular were, up to several years ago, considered the ٘forgotten brain circuitry. The subsequent release of glial stressors or activat- cells,ٙ or neglected stepchildren of neuroscience, now act as ing signals contributes to neuropathic pain and neuroinflam- orchestrators in the tetrapartite synapse, and control of their mation. Recent studies document the importance of glia in activation state is crucial to the integrity of CNS function. the development and persistence of neuropathic pain and Recent technical advances and increased interest in elucidat- neuroinflammation as a connecting link, thereby focusing at- ing the role of non-neuronal cells of the CNS in both the tention on the glial pathology as the general underlying fac- physiological and pathophysiologial processes have cata- tor in essentially all age-related neurodegenerative diseases. There is wide agreement that excessive glial activation is a pulted these cells into the forefront of neuroscience research. key process in nervous system disorders involving the re- Glial cells do not conduct nerve impulses, and they provide lease of strong pro-inflammatory cytokines, which can trig- structural support for the brain, assisting in nervous system ger worsening of multiple disease states. -

Glial Repair in an Insect Central Nervous System: Effects of Surgical Lesioning’
0270-6474/84/0411-2689$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 11, pp. 2689-2697 Printed in U.S.A. November 1984 GLIAL REPAIR IN AN INSECT CENTRAL NERVOUS SYSTEM: EFFECTS OF SURGICAL LESIONING’ J. E. TREHERNE,2 J. B. HARRISON, J. M. TREHERNE, AND N. J. LANE Agricultural Food Reserve Council Unit of Insect Neurophysiology and Pharmacology, Department of Zoology, University of Cambridge, CB2 3EJ United Kingdom Received December 27, 1983; Revised April 23, 1984; Accepted May 8, 1984 Abstract Surgical lesioning of central nervous connectives in the cockroach (Periplaneta americana (L.)), although causing only local glial damage, resulted in complex and prolonged cellular changes. An early response to mechanical disruption was the appearance of granule-containing cells within the damaged perineurium, among adjacent,, undamaged, perineurial cells, and between glial processes deep within the connectives. These cells, which were strikingly similar to hemocytes, were clearly involved in phagocytic activity and persisted in the damaged regions for more than a month after lesioning. There was only a slow restoration of organized perineurial glia and re-establishment of the blood-brain barrier, as indicated by the exclusion of an extracellular tracer, ionic lanthanum. These observations contrast with the speedy, ordered repair of the neuroglia observed following selective glial disruption and suggest that undamaged axons and/or the extracellular matrix exert a profound influence on the mechanisms of glial repair. It is now increasingly recognized that the supporting cells of has a number of advantages: notably, relative structural sim- the brain, the neuroglia, serve a number of important physio- plicity, its accessibility to electrophysiological study, and the logical roles. -
Nervous System - Neurons
Nervous System - Neurons Biol 105 Chapter 7 Outline I. Nervous system function II. Central and peripheral nervous system III. Nervous system cells IV. Myelinated neurons V. Nerve signal transmission VI. Nerve Synapse Copyright © 2009 Pearson Education, Inc. Nervous Tissues . Nervous tissue functions to conduct messages throughout the body. When nerve cells are stimulated, an electrical signal quickly travels through the nerve cell to the nerve ending, triggering events. Copyright © 2009 Pearson Education, Inc. Nervous System . Includes nervous tissue and sensory organs. Nervous system functions to: . Sense the environment – it receives information from both outside and inside the body. Process the information it receives. Respond to information – send out orders. Copyright © 2009 Pearson Education, Inc. Two Parts of the Nervous System 1. Central Nervous System (CNS) . Brain and Spinal Cord. 2. Peripheral Nervous System (PNS) . Nervous tissue outside brain and spine. Sense organs. Copyright © 2009 Pearson Education, Inc. Central Nervous System Peripheral Copyright © 2009 Pearson Education, Inc. Figure 8.1 The nervous system Copyright © 2009 Pearson Education, Inc. Nervous System Cells . Two types of nervous tissue cells. Neurons – The cells that are responsible for transmitting messages. Neuroglial Cells – Cells that support the neurons. Copyright © 2009 Pearson Education, Inc. Neuroglial Cells . Microglia – Immune system cells, engulf bacteria and cellular debris. Astrocytes – Provide nutrients to neurons. Oligodenrocytes and Schwann Cells – Form myelin sheaths. Copyright © 2009 Pearson Education, Inc. Copyright © 2009 Pearson Education, Inc. Parts of a Neuron . Cell body – contains the nucleus, main body of cell. Dendrites – projections from the cell body that carry messages to the cell body. Axon – one projection that carries messages away from the cell body (can be very long). -
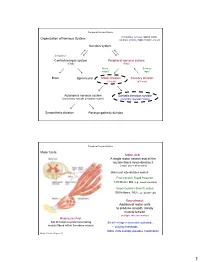
Nervous System Central Nervous System Peripheral Nervous System
Peripheral Nervous System Involuntary reflexes (spinal cord); Organization of Nervous System: voluntary actions (higher brain centers) Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (efferent) (afferent) Autonomic nervous system Somatic nervous system (involuntary; smooth & cardiac muscle) (voluntary; skeletal muscle) Sympathetic division Parasympathetic division Peripheral Nervous System Motor Units: Motor Unit: A single motor neuron and all the muscle fibers innervated by it (motor unit = all-or-none) Motor unit size dictates control: Fine Control / Rapid Reaction: 1-10 fibers / MU (e.g., ocular muscles) Gross Control / Slow Reaction: 1000’s fibers / MU (e.g., quadriceps) Recruitment: Addition of motor units to produce smooth, steady muscle tension (multiple fiber summation) Motoneuron Pool: Set of motor neurons innervating Small large motor units activated… muscle fibers within the same muscle • Varying thresholds Motor units overlap; provides coordination Marieb & Hoehn – Figure 9.13 1 Peripheral Nervous System Types of Motor Neurons: 1) Alpha () motor neurons: • Give rise to large Type A alpha (A) motor nerve fibers (~ 14 µm diameter) • Innervate extrafusal skeletal muscle fibers (generate force) 2) Gamma () motor neurons: • Give rise to small Type A gamma (Aγ) motor nerve fibers (~ 5 µm diameter) • Innervate intrafusal muscle fibers (small, specialized fibers – muscle spindle) What is the length of the muscle? Proper -

The Central Nervous System
U.S. ARMY MEDICAL DEPARTMENT CENTER AND SCHOOL FORT SAM HOUSTON, TEXAS 78234-6100 THE CENTRAL NERVOUS SYSTEM SUBCOURSE MD0572 EDITION 100 DEVELOPMENT This subcourse is approved for resident and correspondence course instruction. It reflects the current thought of the Academy of Health Sciences and conforms to printed Department of the Army doctrine as closely as currently possible. Development and progress render such doctrine continuously subject to change. The subject matter expert responsible for content accuracy of this edition was the NCOIC, Nursing Science Division, DSN 471-3086 or area code (210) 221-3086, M6 Branch, Academy of Health Sciences, ATTN: MCCS-HNP, Fort Sam Houston, Texas 78234-6100. ADMINISTRATION Students who desire credit hours for this correspondence subcourse must meet eligibility requirements and must enroll in the subcourse. Application for enrollment should be made at the Internet website: http://www.atrrs.army.mil. You can access the course catalog in the upper right corner. Enter School Code 555 for medical correspondence courses. Copy down the course number and title. To apply for enrollment, return to the main ATRRS screen and scroll down the right side for ATRRS Channels. Click on SELF DEVELOPMENT to open the application and then follow the on screen instructions. For comments or questions regarding enrollment, student records, or examination shipments, contact the Nonresident Instruction Branch at DSN 471-5877, commercial (210) 221-5877, toll-free 1-800-344-2380; fax: 210-221-4012 or DSN 471-4012, e-mail [email protected], or write to: NONRESIDENT INSTRUCTION BRANCH AMEDDC&S ATTN: MCCS-HSN 2105 11TH STREET SUITE 4191 FORT SAM HOUSTON TX 78234-5064 CLARIFICATION OF TERMINOLOGY When used in this publication, words such as "he," "him," "his," and "men" 'are intended to include both the masculine and feminine genders, unless specifically stated otherwise or when obvious in context. -

MUSCLE STRETCH REFLEX: Components and Process Description
MUSCLE STRETCH REFLEX: Components and Process Description Introduction The muscle stretch reflex is an unconscious action caused by the collaboration between a person’s nervous and muscular systems. The reflex acts to prevent damage to muscles and maintain sensory input to the central nervous system. Often, these reflexes are tested during check-ups to make sure there are no problems with the patient’s nervous and muscular systems. The reflex happens when a muscle is stretched and causes an unconscious contraction of the stretched muscles to prevent injury. To describe the process, this description will be looking at the knee jerk reflex and will explain how muscle spindles regulate such a reflex. Muscle Spindle Location and Components Muscle spindles are arranged within whole muscle; parallel to the muscle fibers. As seen in Figure 1, there are many components involved in muscle stretch reflex. • Extrafusal muscle fibers: These are the normal, contractile muscle fibers found in skeletal muscles. These fibers are innervated by alpha motor neurons (not shown in Figure 1). • Muscle spindle: This is the Figure 1: Muscle Spindle sensory and regulatory organ involved in the muscle stretch reflex. It is arranged within muscles; parallel to the muscle fibers. There are various components that make up a muscle spindle. These components include: o The central region: This is the middle part of the muscle spindle. The central region lacks myofibrils and is noncontractile. This region contains the ends of a sensory afferent neuron. o The sensory afferent neuron: This is a tonically active (always firing action potentials) sensory neuron that relays information from the muscle spindle to the central nervous system.