The Effect of Taste and Temperature on Lingual Swallowing Pressure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
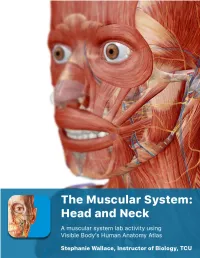
The Muscular System Views
1 PRE-LAB EXERCISES Before coming to lab, get familiar with a few muscle groups we’ll be exploring during lab. Using Visible Body’s Human Anatomy Atlas, go to the Views section. Under Systems, scroll down to the Muscular System views. Select the view Expression and find the following muscles. When you select a muscle, note the book icon in the content box. Selecting this icon allows you to read the muscle’s definition. 1. Occipitofrontalis (epicranius) 2. Orbicularis oculi 3. Orbicularis oris 4. Nasalis 5. Zygomaticus major Return to Muscular System views, select the view Head Rotation and find the following muscles. 1. Sternocleidomastoid 2. Scalene group (anterior, middle, posterior) 2 IN-LAB EXERCISES Use the following modules to guide your exploration of the head and neck region of the muscular system. As you explore the modules, locate the muscles on any charts, models, or specimen available. Please note that these muscles act on the head and neck – those that are located in the neck but act on the back are in a separate section. When reviewing the action of a muscle, it will be helpful to think about where the muscle is located and where the insertion is. Muscle physiology requires that a muscle will “pull” instead of “push” during contraction, and the insertion is the part that will move. Imagine that the muscle is “pulling” on the bone or tissue it is attached to at the insertion. Access 3D views and animated muscle actions in Visible Body’s Human Anatomy Atlas, which will be especially helpful to visualize muscle actions. -

Questions on Human Anatomy
Standard Medical Text-books. ROBERTS’ PRACTICE OF MEDICINE. The Theory and Practice of Medicine. By Frederick T. Roberts, m.d. Third edi- tion. Octavo. Price, cloth, $6.00; leather, $7.00 Recommended at University of Pennsylvania. Long Island College Hospital, Yale and Harvard Colleges, Bishop’s College, Montreal; Uni- versity of Michigan, and over twenty other medical schools. MEIGS & PEPPER ON CHILDREN. A Practical Treatise on Diseases of Children. By J. Forsyth Meigs, m.d., and William Pepper, m.d. 7th edition. 8vo. Price, cloth, $6.00; leather, $7.00 Recommended at thirty-five of the principal medical colleges in the United States, including Bellevue Hospital, New York, University of Pennsylvania, and Long Island College Hospital. BIDDLE’S MATERIA MEDICA. Materia Medica, for the Use of Students and Physicians. By the late Prof. John B Biddle, m.d., Professor of Materia Medica in Jefferson Medical College, Phila- delphia. The Eighth edition. Octavo. Price, cloth, $4.00 Recommended in colleges in all parts of the UnitedStates. BYFORD ON WOMEN. The Diseases and Accidents Incident to Women. By Wm. H. Byford, m.d., Professor of Obstetrics and Diseases of Women and Children in the Chicago Medical College. Third edition, revised. 164 illus. Price, cloth, $5.00; leather, $6.00 “ Being particularly of use where questions of etiology and general treatment are concerned.”—American Journal of Obstetrics. CAZEAUX’S GREAT WORK ON OBSTETRICS. A practical Text-book on Midwifery. The most complete book now before the profession. Sixth edition, illus. Price, cloth, $6.00 ; leather, $7.00 Recommended at nearly fifty medical schools in the United States. -

Facial-Stapedial Synkinesis Following Acute Idiopathic Facial Palsy
CASE REPORT Facial-Stapedial Synkinesis Following Acute Idiopathic Facial Palsy Michael Hutz, MD; Margaret Aasen; John Leonetti, MD ABSTRACT complete resolution of their unilateral Introduction: While most patients note a complete resolution of facial paralysis in Bell’s Palsy, facial paralysis, the remaining patients up to 30% will have persistent facial weakness and develop synkinesis. All branches of the manifest persistent paralysis or develop facial nerve are at risk for developing synkinesis, but stapedial synkinesis has rarely been synkinesis, which occurs when a volun- reported in the literature. tary muscle movement causes a simulta- Case Presentation: A 45-year-old man presented with sudden onset, complete right facial neous involuntary contraction of other paralysis. One-and-a-half years later, he had persistent facial weakness and synkinesis. He muscles. The facial nerve is the 7th cra- noted persistent right aural fullness and hearing loss. Audiometry demonstrated facial-stapedial nial nerve and is primarily affected in synkinesis. Bell’s Palsy. It acts to control the muscles Discussion: The patient was diagnosed with stapedial synkinesis based on audiometric find- of facial expression and conveys taste sen- ings by comparing his hearing at rest and with sustained facial mimetic movement. A literature sation to the anterior two-thirds of the review revealed 21 reported cases of this disorder. tongue. Faulty facial nerve regeneration fol- Conclusions: Facial-stapedial synkinesis is an underdiagnosed phenomenon for patients recov- ering from idiopathic facial palsy. Patients who develop facial synkinesis also may have a com- lowing Bell’s Palsy commonly leads to ponent of stapedial synkinesis and should be referred to an otolaryngologist if they complain abnormal muscle contractions of the eye, of any otologic symptoms, such as unilateral hearing loss or tinnitus. -

EDS Awareness in the TMJ Patient
EDS Awareness in the TMJ Patient TMJ and CCI with the EDS Patient “The 50/50” Myofascial Pain Syndrome EDNF, Baltimore, MD August 14,15, 2015 Generation, Diagnosis and Treatment of Head Pain of Musculoskeletal Origin Head pain generated by: • Temporomandibular joint dysfunction • Cervicocranial Instability • Mandibular deviation • Deflection of the Pharyngeal Constrictor Structures Parameters & Observations . The Myofascial Pain Syndrome (MPS) is a description of pain tracking in 200 Ehlers-Danlos patients. Of the 200 patients, 195 were afflicted with this pain referral syndrome pattern. The MPS is in direct association and correlation to Temporomandibular Joint dysfunction and Cervico- Cranial Instability syndromes. Both syndromes are virtually and always correlated. Evaluation of this syndrome was completed after testing was done to rule out complex or life threatening conditions. The Temporomandibular Joint TMJ Dysfunction Symptoms: Deceptively Simple, with Complex Origins 1) Mouth opening, closing with deviation of mandibular condyles. -Menisci that maybe subluxated causing mandibular elevation. -Jaw locking “open” or “closed”. -Inability to “chew”. 2) “Headaches”/”Muscles spasms” (due to decreased vertical height)generated in the temporalis muscle, cheeks areas, under the angle of the jaw. 3) Osseous distortion Pain can be generated in the cheeks, floor of the orbits and/or sinuses due to osseous distortion associated with “bruxism”. TMJ dysfunction cont. (Any of the following motions may produce pain) Pain With: . Limited opening(closed lock): . Less than 33 mm of rotation in either or both joints . Translation- or lack of . Deviations – motion of the mandible to the affected side or none when both joints are affected . Over joint pain with or without motion around or . -

The Myloglossus in a Human Cadaver Study: Common Or Uncommon Anatomical Structure? B
Folia Morphol. Vol. 76, No. 1, pp. 74–81 DOI: 10.5603/FM.a2016.0044 O R I G I N A L A R T I C L E Copyright © 2017 Via Medica ISSN 0015–5659 www.fm.viamedica.pl The myloglossus in a human cadaver study: common or uncommon anatomical structure? B. Buffoli*, M. Ferrari*, F. Belotti, D. Lancini, M.A. Cocchi, M. Labanca, M. Tschabitscher, R. Rezzani, L.F. Rodella Section of Anatomy and Physiopathology, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy [Received: 1 June 2016; Accepted: 18 July 2016] Background: Additional extrinsic muscles of the tongue are reported in literature and one of them is the myloglossus muscle (MGM). Since MGM is nowadays considered as anatomical variant, the aim of this study is to clarify some open questions by evaluating and describing the myloglossal anatomy (including both MGM and its ligamentous counterpart) during human cadaver dissections. Materials and methods: Twenty-one regions (including masticator space, sublin- gual space and adjacent areas) were dissected and the presence and appearance of myloglossus were considered, together with its proximal and distal insertions, vascularisation and innervation. Results: The myloglossus was present in 61.9% of cases with muscular, ligamen- tous or mixed appearance and either bony or muscular insertion. Facial artery pro- vided myloglossal vascularisation in the 84.62% and lingual artery in the 15.38%; innervation was granted by the trigeminal system (buccal nerve and mylohyoid nerve), sometimes (46.15%) with hypoglossal component. Conclusions: These data suggest us to not consider myloglossus as a rare ana- tomical variant. -

MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients After Whiplash Injury
Journal of Clinical Medicine Article MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury Yeon-Hee Lee 1,* , Kyung Mi Lee 2 and Q-Schick Auh 1 1 Department of Orofacial Pain and Oral Medicine, Kyung Hee University Dental Hospital, #613 Hoegi-dong, Dongdaemun-gu, Seoul 02447, Korea; [email protected] 2 Department of Radiology, Kyung Hee University College of Medicine, Kyung Hee University Hospital, #26 Kyunghee-daero, Dongdaemun-gu, Seoul 02447, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-2-958-9409; Fax: +82-2-968-0588 Abstract: Objective: to investigate the change in volume and signal in the masticatory muscles and temporomandibular joint (TMJ) of patients with temporomandibular disorder (TMD) after whiplash injury, based on magnetic resonance imaging (MRI), and to correlate them with other clinical parameters. Methods: ninety patients (64 women, 26 men; mean age: 39.36 ± 15.40 years), including 45 patients with symptoms of TMD after whiplash injury (wTMD), and 45 age- and sex- matched controls with TMD due to idiopathic causes (iTMD) were included. TMD was diagnosed using the study diagnostic criteria for TMD Axis I, and MRI findings of the TMJ and masticatory muscles were investigated. To evaluate the severity of TMD pain and muscle tenderness, we used a visual analog scale (VAS), palpation index (PI), and neck PI. Results: TMD indexes, including VAS, PI, and neck PI were significantly higher in the wTMD group. In the wTMD group, muscle tenderness was highest in the masseter muscle (71.1%), and muscle tenderness in the temporalis (60.0%), lateral pterygoid muscle (LPM) (22.2%), and medial pterygoid muscle (15.6%) was significantly more frequent than that in the iTMD group (all p < 0.05). -
The Mandibular Nerve - Vc Or VIII by Prof
The Mandibular Nerve - Vc or VIII by Prof. Dr. Imran Qureshi The Mandibular nerve is the third and largest division of the trigeminal nerve. It is a mixed nerve. Its sensory root emerges from the posterior region of the semilunar ganglion and is joined by the motor root of the trigeminal nerve. These two nerve bundles leave the cranial cavity through the foramen ovale and unite immediately to form the trunk of the mixed mandibular nerve that passes into the infratemporal fossa. Here, it runs anterior to the middle meningeal artery and is sandwiched between the superior head of the lateral pterygoid and tensor veli palatini muscles. After a short course during which a meningeal branch to the dura mater, and the nerve to part of the medial pterygoid muscle (and the tensor tympani and tensor veli palatini muscles) are given off, the mandibular trunk divides into a smaller anterior and a larger posterior division. The anterior division receives most of the fibres from the motor root and distributes them to the other muscles of mastication i.e. the lateral pterygoid, medial pterygoid, temporalis and masseter muscles. The nerve to masseter and two deep temporal nerves (anterior and posterior) pass laterally above the medial pterygoid. The nerve to the masseter continues outward through the mandibular notch, while the deep temporal nerves turn upward deep to temporalis for its supply. The sensory fibres that it receives are distributed as the buccal nerve. The 1 | P a g e buccal nerve passes between the medial and lateral pterygoids and passes downward and forward to emerge from under cover of the masseter with the buccal artery. -

Atlas of the Facial Nerve and Related Structures
Rhoton Yoshioka Atlas of the Facial Nerve Unique Atlas Opens Window and Related Structures Into Facial Nerve Anatomy… Atlas of the Facial Nerve and Related Structures and Related Nerve Facial of the Atlas “His meticulous methods of anatomical dissection and microsurgical techniques helped transform the primitive specialty of neurosurgery into the magnificent surgical discipline that it is today.”— Nobutaka Yoshioka American Association of Neurological Surgeons. Albert L. Rhoton, Jr. Nobutaka Yoshioka, MD, PhD and Albert L. Rhoton, Jr., MD have created an anatomical atlas of astounding precision. An unparalleled teaching tool, this atlas opens a unique window into the anatomical intricacies of complex facial nerves and related structures. An internationally renowned author, educator, brain anatomist, and neurosurgeon, Dr. Rhoton is regarded by colleagues as one of the fathers of modern microscopic neurosurgery. Dr. Yoshioka, an esteemed craniofacial reconstructive surgeon in Japan, mastered this precise dissection technique while undertaking a fellowship at Dr. Rhoton’s microanatomy lab, writing in the preface that within such precision images lies potential for surgical innovation. Special Features • Exquisite color photographs, prepared from carefully dissected latex injected cadavers, reveal anatomy layer by layer with remarkable detail and clarity • An added highlight, 3-D versions of these extraordinary images, are available online in the Thieme MediaCenter • Major sections include intracranial region and skull, upper facial and midfacial region, and lower facial and posterolateral neck region Organized by region, each layered dissection elucidates specific nerves and structures with pinpoint accuracy, providing the clinician with in-depth anatomical insights. Precise clinical explanations accompany each photograph. In tandem, the images and text provide an excellent foundation for understanding the nerves and structures impacted by neurosurgical-related pathologies as well as other conditions and injuries. -

Appendix B: Muscles of the Speech Production Mechanism
Appendix B: Muscles of the Speech Production Mechanism I. MUSCLES OF RESPIRATION A. MUSCLES OF INHALATION (muscles that enlarge the thoracic cavity) 1. Diaphragm Attachments: The diaphragm originates in a number of places: the lower tip of the sternum; the first 3 or 4 lumbar vertebrae and the lower borders and inner surfaces of the cartilages of ribs 7 - 12. All fibers insert into a central tendon (aponeurosis of the diaphragm). Function: Contraction of the diaphragm draws the central tendon down and forward, which enlarges the thoracic cavity vertically. It can also elevate to some extent the lower ribs. The diaphragm separates the thoracic and the abdominal cavities. 2. External Intercostals Attachments: The external intercostals run from the lip on the lower border of each rib inferiorly and medially to the upper border of the rib immediately below. Function: These muscles may have several functions. They serve to strengthen the thoracic wall so that it doesn't bulge between the ribs. They provide a checking action to counteract relaxation pressure. Because of the direction of attachment of their fibers, the external intercostals can raise the thoracic cage for inhalation. 3. Pectoralis Major Attachments: This muscle attaches on the anterior surface of the medial half of the clavicle, the sternum and costal cartilages 1-6 or 7. All fibers come together and insert at the greater tubercle of the humerus. Function: Pectoralis major is primarily an abductor of the arm. It can, however, serve as a supplemental (or compensatory) muscle of inhalation, raising the rib cage and sternum. (In other words, breathing by raising and lowering the arms!) It is mentioned here chiefly because it is encountered in the dissection. -

The Muscles of the Jaw Are Some of the Strongest in the Human Body
MUSCLES OF MASTICATION The muscles of the jaw are some of the strongest in the human body. They aid in chewing and speech by allowing us to open and close our mouths. Ready to unlock the mysteries of mastication? Then read on! OF MASSETERS AND MANDIBLES The deep and superficial masseter muscles enable mastication (chewing by pulling the mandible (jawbone) up towards the maxillae. Factoid! Humans’ jaws are able to bite with DEEP a force of about 150-200 psi (890 MASSETER Newtons). In contrast, a saltwater crocodile can bite with a force of 3,700 SUPERFICIAL psi (16, 400 Newtons)! MASSETER MAXILLA (R) 2 MANDIBLE MORE MASSETER FACTS The deep masseter’s origin is the zygomatic arch and the superficial masseter’s origin is the zygomatic bone. Both masseters insert into the ramus of the mandible, though the deep masseter’s insertion point is closer to the temporomandibular joint. The mandible is the only bone in the skull that we can consciously move (with the help of muscles, of course). 3 TEMPORALIS The temporalis muscles sit on either side of the head. Their job is to elevate and retract the mandible against the maxillae. They originate at the temporal fossa and temporal fascia and insert at the coronoid process and ramus of the mandible. 4 LATERAL SUPERIOR PTERYGOIDS HEAD The lateral pterygoids draw the mandibular condyle and articular disc of the temporomandibular joint forward. Each lateral pterygoid has two heads. The superior head originates at the sphenoid and infratemporal crest and the inferior head originates at the lateral pterygoid plate. -

Of the One-Humped Camel
J. Anat. (1970), 106, 2, pp. 341-348 341 With 3 figures Printed in Great Britain The course and branches of the facial nerve of the one-humped camel I. ARNAUTOVIC, M. E. ABU SINEINA AND M. STANIC Faculty of Veterinary Science, University of Khartoum (Received 14 February 1969) The course and ramification of the facial nerve of the camel have not previously been clearly established, and the brief references that occur in the literature are of a rather general kind. Thus Lesbre (1906) stated that the cranial nerves of the camel were similar to those of ruminants, and Leese (1927) concluded that there were no significant differences between the course of the facial nerve of the camel and that of other ruminants. Droandi (1936) gave a more detailed account of the facial nerve which he described as ramifying on the external surface of the head. Tayeb (1958), who studied the cranial nerves of the camel, did not give the full account of the facial nerve. MATERIAL AND METHODS During the period July 1966 to December 1967 the heads of fifteen camels slaugh- tered at Tamboul Slaughterhouse, south-east of Khartoum, were collected for dis- section at the Faculty of Veterinary Science, Shambat. The heads belonged to normal healthy animals, seven males and eight females, varying in age from 4 to 10 years. Both sides of each head were used in the study. The heads were removed, with their skin intact, from the carcasses at the level of the third cervical vertebra. Some of the specimens were dissected immediately after collection, others were injected with 10 % formalin and studied later. -

Muscles of Mastication Muscles That Move the Head
1 Muscles Of Mastication identification origin insertion action maxilla, zygomatic arch Mandible elevates & protracts mandible MASSETER Human Cat Zygomatic Bone Mandible elevates mandible TEMPORALIS Human/Cat Temporal Bone Mandible elevates and retracts mandible Hyoid Bone DIGASTRIC Human mandible & mastoid process depress mandible Cat occipital bone & mastoid process Mandible depress mandible raises floor of mouth; MYLOHYOID Human/Cat Mandible Hyoid bone pulls hyoid forward Muscles That Move The Head identification origin insertion action STERNOCLEIDOMAStoID clavicle, sternum mastoid process flexes and laterally rotates head HUMAN ONLY STERNOMAStoID CAT ONLY sternum mastoid process turns and depresses head pulls head laterally; CLEIDOMAStoID CAT ONLY clavicle mastoid process pulls clavicle craniad 2 Muscles Of The Hyoid, Larynx And Tongue identification origin insertion action Human Sternum Hyoid depresses hyoid bone STERNOHYOID Cat costal cartilage 1st rib Hyoid pulls hyoid caudally; raises ribs and sternum sternum Throid cartilage of larynx Human depresses thyroid cartilage STERNothYROID Cat costal cartilage 1st rib Throid cartilage of larynx pulls larynx caudad elevates thyroid cartilage and Human thyroid cartilage of larynx Hyoid THYROHYOID depresses hyoid bone Cat thyroid cartilage of larynx Hyoid raises larynx GENIOHYOID Human/Cat Mandible Hyoid pulls hyoid craniad 3 Muscles That Attach Pectoral Appendages To Vertebral Column identification origin insertion action Human Occipital bone; Thoracic and Cervical raises clavicle; adducts,