Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Formulation and Evaluation of Transdermal Patch and Gel of Nateglinide
Human Journals Research Article September 2015 Vol.:4, Issue:2 © All rights are reserved by C. Aparna et al. Formulation and Evaluation of Transdermal Patch and Gel of Nateglinide Keywords: Nateglinide, transdermal patch and gel, HPMC, ethyl cellulose, carbopol, PVA, PVP ABSTRACT Anusha Gundeti, C. Aparna*, Dr. Prathima Srinivas The objective of the present work was to formulate Transdermal Drug Delivery systems of Nateglinide, an Department of Pharmaceutics, Sri Venkateshwara antidiabetic drug belonging to meglitinide class with a half life of 1.5 hrs. Transdermal patches containing nateglinide were College of Pharmacy, prepared by solvent casting method using the combinations Affiliated to Osmania University, of HPMC:EC, PVA:PVP, HPMC:Eudragit RS 100, Eudragit RL100:RS100 in different proportions and by incorporating Madhapur, Hyderabad, Telangana -500081, India. different permeation enhancers (polyethylene glycol 400, Su bmission: 7 September 2015 DMSO). The transdermal patches were evaluated for their physicochemical properties like thickness, weight variation, Accepted: 11 September 2015 folding endurance, percentage moisture absorption, percentage Published: 25 September 2015 moisture loss, in-vitro diffusion studies & ex-vivo permeation studies. Transdermal Gel was formulated using HPMC, carbopol 934, carbopol 940 and methyl cellulose. Gels were evaluated for homogeneity, pH, viscosity, drug content, in-vitro diffusion studies & ex-vivo permeation studies. By comparing the drug release F5 (HPMC:EC) formulation was selected as optimized formulation as it could sustain the drug release for 12 hrs i.e. 99.2% when compared to gel. Stability studies were www.ijppr.humanjournals.com carried out according to ICH guidelines and the patches maintained integrity and good physicochemical properties during the study period. -
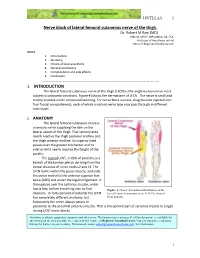
Nerve Block of Lateral Femoral Cutaneous Nerve of the Thigh
18VTLLAA 1 Nerve block of lateral femoral cutaneous nerve of the thigh. Dr. Robert M Raw (MD) . MBChB, MFGP, MPraxMed, DA, FCA. Professor of Anesthesia retired Editor of Regional-Anesthesia.Com INDEX. 1. Introduction 2. Anatomy 3. Choice of local anesthetic 4. General indications 5. Complications and side effects 6. Conclusion ------------------------------------------------------------------------------------ 1. INTRODUCTION The lateral femoral cutaneous nerve of the thigh (LFCN) is the single human nerve most subject to anatomic variations. Figure #1shows the dermatome of LFCN. The nerve is small and mostly invisible under ultrasound scanning. For nerve block success, drug must be injected into four fascial compartments, each of which a variant nerve type may pass through in different individuals. 2. ANATOMY The lateral femoral cutaneous nerve is a sensory nerve supplying the skin on the lateral aspect of the thigh. That sensory area nearly reaches the thigh posterior midline and the thigh anterior midline. Its superior limit passes over the greater trochanter and its inferior limit nearly reaches the height of the patella. The typical LCNT, in 60% of patients, is a branch of the lumbar plexus deriving from the dorsal divisions of nerve roots L2 and L3. The LFCN forms within the psoas muscle, and exits the pelvis medial to the anterior superior iliac spine (ASIS) and under the inguinal ligament. It then passes over the sartorius muscle, under fascia lata, before branching into its final Figure 1. Classic dermatomal distribution of the divisions. In forty percent of patients the LFCN lateral femoral cutaneous nerve (LFCN), derived has completely different anatomy, but from Sobotta. fortunately the nerve always passes in proximity to the proximal sartorius muscle. -

Transdermal Absorption Preparation
Europäisches Patentamt *EP001522316A1* (19) European Patent Office Office européen des brevets (11) EP 1 522 316 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 158(3) EPC (43) Date of publication: (51) Int Cl.7: A61K 47/34, A61K 47/10, 13.04.2005 Bulletin 2005/15 A61K 47/14, A61K 9/06, A61K 9/08, A61K 9/12, (21) Application number: 03764126.3 A61K 9/70 (22) Date of filing: 02.07.2003 (86) International application number: PCT/JP2003/008400 (87) International publication number: WO 2004/006960 (22.01.2004 Gazette 2004/04) (84) Designated Contracting States: • OMICHI, Katsuhiro AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Saitama-shi, Saitama 338-0832 (JP) HU IE IT LI LU MC NL PT RO SE SI SK TR • OKADA, Minoru Designated Extension States: Inzai-shi, Chiba 270-1323 (JP) AL LT LV MK • KURAZUMI, Toshiaki Narita-shi, Chiba 286-0011 (JP) (30) Priority: 16.07.2002 JP 2002206565 (74) Representative: Hartz, Nikolai F., Dr. (71) Applicant: SSP Co., Ltd. Wächtershäuser & Hartz Chuo-ku, Tokyo 103-8481 (JP) Patentanwälte Weinstrasse 8 (72) Inventors: 80333 München (DE) • NARUI, Takashi Sakura-shi, Chiba 285-0817 (JP) (54) TRANSDERMAL ABSORPTION PREPARATION (57) A transdermal absorption promotion composi- and transdermal absorption preparation not only exhibit tion comprising the following components (a), (b), and an excellent transdermal absorption promotion effect, (c) and a transdermal absorption preparation compris- but also exhibit superior skin-permeability, even if a drug ing the following components (a), (b), (c), and (d) are having a relatively high lipophilic property and poor disclosed. -

Preparatory: 1 Venous Access and Medication Administration: 4
Preparatory: 1 Venous Access and Medication Administration: 4 W4444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444 UNIT TERMINAL OBJECTIVE 1-4 At the completion of this unit, the EMT-Critical Care Technician student will be able to safely and precisely access the venous circulation and administer medications. COGNITIVE OBJECTIVES At the completion of this unit, the EMT-Critical Care Technician student will be able to: 1-4.1 Review the specific anatomy and physiology pertinent to medication administration. (C-1) 1-4.2 Review mathematical principles. (C-1) 1-4.3 Review mathematical equivalents. (C-1) 1-4.4 Differentiate temperature readings between the Centigrade and Fahrenheit scales. (C-3) 1-4.5 Discuss formulas as a basis for performing drug calculations. (C-1) 1-4.6 Calculate oral and parenteral drug dosages for all emergency medications administered to adults, infants and children. (C-2) 1-4.7 Calculate intravenous infusion rates for adults, infants, and children. (C-2) 1-4.8 Discuss legal aspects affecting medication administration. (C-1) 1-4.9 Discuss the "six rights" of drug administration and correlate these with the principles of medication administration. (C-1) 1-4.10 Discuss medical asepsis and the differences between clean and sterile techniques. (C-1) 1-4.11 Describe use of antiseptics and disinfectants. (C-1) 1-4.12 Describe the use of universal precautions and body substance isolation (BSI) procedures when administering a medication. (C-1) 1-4.13 Describe the indications, equipment needed, techniques utilized, precautions, and general principles of peripheral venous cannulation (Including saline locks). (C-1) 1-4.14 Describe the indications, equipment needed, techniques utilized, precautions, and general principles of intraosseous needle placement and infusion. -

Handbook ESRA
TECHNIQUES HEAD & NECK 4 Intracranial surgery p. 3 Eye blocks p. 5 Face anatomy p. 16 Face particularity p. 23 Ophtalmic nerve blocks p. 27 Maxillary nerve blocks p. 33 Mandibular nerve blocks p. 46 THORAX & ABDOMEN 50 Epidural anaesthesia in Cardio-thoracic surgery p. 50 Ilioinguinal-Iliohypogastric block p. 55 Peri-umbilical & Rectus sheath block p. 57 Pudendal block p. 58 UPPER LIMB 61 Choice of a technique p. 61 Brachial plexus anatomy p. 65 Interscalen block p. 68 Supraclavicular blocks p. 73 Infraclavicular blocks p. 80 Axillary block p. 83 LOWER LIMB 90 Lumbar plexus block p. 90 Iliofascial block p. 100 Obturator block p. 102 Sciatic blocks o Sciatic blocks - parasacral nerve approach p. 109 o Sciatic blocks - posterior popliteal approach p. 115 Ankle blocks p. 119 AXIAL BLOCKS 123 Lumbar epidural p. 123 OBSTETRICS AXIAL BLOCKS 126 Epidural p. 126 PERIPHERAL BLOCKS Pudendal block p. 58 2 Aknowledgement The provenience of the materials included in this handbook is from the Learning Zone on the official site of “European Society of Regional Anesthesia and Pain Therapy”. http://www.esra-learning.com/ 2007 3 HEAD & TABLE OF CONTENTS NECK • Intracranial surgery • Eye blocks • Face anatomy • Face particularity • Ophtalmic nerve blocks • Maxillary nerve blocks • Mandibular nerve blocks • Cervical plexus blocks HEAD & INTRACRANIAL SURGERY NECK Paul J. Zetlaoui, M.D. Kremlin-Bicetre - France In intra-cranial neurosurgery, scalp infiltration aims to prevent systematic and cerebral hemodynamic variations, contemporary of skin incision. The potential morbidity of these hypertension-tachycardia episodes, even in patients profoundly anaesthetized, is secondary in the increase of the cerebral blood flow and in its deleterious consequences on intra-cranial pressure in these compromised patients. -

The Development of an Intramuscular Injection Simulation for Nursing Students
Open Access Technical Report DOI: 10.7759/cureus.12366 The Development of an Intramuscular Injection Simulation for Nursing Students Julia Micallef 1 , Artur Arutiunian 1 , Adam Dubrowski 1 1. Health Sciences, Ontario Tech University, Oshawa, CAN Corresponding author: Adam Dubrowski, [email protected] Abstract Intramuscular (IM) injections are preferred over subcutaneous injections for administering medicine such as epinephrine and vaccines as the muscle tissue contains an increased vascular supply that provides ideal absorption of the drug being administered. However, administering an IM injection requires clinical judgment when choosing the injection site, understanding the relevant anatomy and physiology as well as the principles and techniques for administering an IM injection. Therefore, it is essential to learn and perform IM injections using injection simulators to practice the skill before administering to a real patient. Current IM injection simulators either favor realism at the expense of standardization or are expensive but do not provide a realistic experience. Therefore, it is imperative to develop an inexpensive but realistic intramuscular injection simulator that can be used to train nursing students so that they can be prepared for when they enter the clinical setting. This technical report aims to provide an overview of the development of an inexpensive and realistic deltoid simulator geared to teach nursing students the skill of IM injections. After development, the IM simulators were tested and validated by practicing nurses. An 18-item survey was administered to the nurses, and results indicated positive feedback about the realism of the simulator, in comparison to previous models used, such as the Wallcur® PRACTI-Injecta Pads (Wallcur LLC, San Diego, CA). -

TOXICOLOGY and EXPOSURE GUIDELINES ______(For Assistance, Please Contact EHS at (402) 472-4925, Or Visit Our Web Site At
(Revised 1/03) TOXICOLOGY AND EXPOSURE GUIDELINES ______________________________________________________________________ (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) "All substances are poisons; there is none which is not a poison. The right dose differentiates a poison and a remedy." This early observation concerning the toxicity of chemicals was made by Paracelsus (1493- 1541). The classic connotation of toxicology was "the science of poisons." Since that time, the science has expanded to encompass several disciplines. Toxicology is the study of the interaction between chemical agents and biological systems. While the subject of toxicology is quite complex, it is necessary to understand the basic concepts in order to make logical decisions concerning the protection of personnel from toxic injuries. Toxicity can be defined as the relative ability of a substance to cause adverse effects in living organisms. This "relative ability is dependent upon several conditions. As Paracelsus suggests, the quantity or the dose of the substance determines whether the effects of the chemical are toxic, nontoxic or beneficial. In addition to dose, other factors may also influence the toxicity of the compound such as the route of entry, duration and frequency of exposure, variations between different species (interspecies) and variations among members of the same species (intraspecies). To apply these principles to hazardous materials response, the routes by which chemicals enter the human body will be considered first. Knowledge of these routes will support the selection of personal protective equipment and the development of safety plans. The second section deals with dose-response relationships. -

An Archetype Swing in Transdermal Drug Delivery
Indo American Journal of Pharmaceutical Research, 2017 ISSN NO: 2231-6876 A COMPREHENSIVE REVIEW ON MICRONEEDLES - AN ARCHETYPE SWING IN TRANSDERMAL DRUG DELIVERY G. Ravi*, N. Vishal Gupta, M. P. Gowrav Department of Pharmaceutics, JSS College of Pharmacy, JSS University, Shri Shivarathreeshwara Nagara, Mysuru, Karnataka, India. ARTICLE INFO ABSTRACT Article history Transdermal drug delivery is the non-invasive delivery of medications through the skin Received 23/12/2016 surface into the systemic circulation. The advantage of transdermal drug delivery system is Available online that it is painless technique of administration of drugs. The advantage of transdermal drug 31/01/2017 delivery system is that it is painless technique of administration of drugs. Transdermal drug delivery system can improve the therapeutic efficacy and safety of the drugs because drug Keywords delivered through the skin at a predetermined and controlled rate. Due to the various Microneedles, biomedical benefits, it has attracted many researches. The barrier nature of stratumcorneum Hypodermic Needles, poses a danger to the drug delivery. By using microneedles, a pathway into the human body Transdermal, can be recognized which allow transportation of macromolecular drugs such as insulin or Stratumcorneum, vaccine. These microneedles only penetrate outer layers of the skin, exterior sufficient not to Patch. reach the nerve receptors of the deeper skin. Thus the microneedles supplement is supposed painless and reduces the infection and injuries. Researches from the past few years showed that microneedles have emerged as a novel carrier and considered to be effective for safe and improved delivery of the different drugs. Microneedles development is created a new pathway in the drug delivery field. -

The Digestive System
69 chapter four THE DIGESTIVE SYSTEM THE DIGESTIVE SYSTEM The digestive system is structurally divided into two main parts: a long, winding tube that carries food through its length, and a series of supportive organs outside of the tube. The long tube is called the gastrointestinal (GI) tract. The GI tract extends from the mouth to the anus, and consists of the mouth, or oral cavity, the pharynx, the esophagus, the stomach, the small intestine, and the large intes- tine. It is here that the functions of mechanical digestion, chemical digestion, absorption of nutrients and water, and release of solid waste material take place. The supportive organs that lie outside the GI tract are known as accessory organs, and include the teeth, salivary glands, liver, gallbladder, and pancreas. Because most organs of the digestive system lie within body cavities, you will perform a dissection procedure that exposes the cavities before you begin identifying individual organs. You will also observe the cavities and their associated membranes before proceeding with your study of the digestive system. EXPOSING THE BODY CAVITIES should feel like the wall of a stretched balloon. With your skinned cat on its dorsal side, examine the cutting lines shown in Figure 4.1 and plan 2. Extend the cut laterally in both direc- out your dissection. Note that the numbers tions, roughly 4 inches, still working with indicate the sequence of the cutting procedure. your scissors. Cut in a curved pattern as Palpate the long, bony sternum and the softer, shown in Figure 4.1, which follows the cartilaginous xiphoid process to find the ventral contour of the diaphragm. -

The Role of Deformable Liposome Characteristics on Skin Permeability of Meloxicam: Optimal Transfersome As Transdermal Delivery Carriers
Send Orders for Reprints to [email protected] The Open Conference Proceedings Journal, 2013, 4, 87-92 87 Open Access The Role of Deformable Liposome Characteristics on Skin Permeability of Meloxicam: Optimal Transfersome as Transdermal Delivery Carriers Sureewan Duangjit1,2, Praneet Opanasopit1, Theerasak Rojarata1, Yasuko Obata2, Yoshinori Oniki2, Kozo Takayama2 and Tanasait Ngawhirunpat1,* 1Department of Pharmaceutical Technology, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand 2Department of Pharmaceutics, Hoshi University, Ebara 2-4-41, Shinagawa-ku, Tokyo 142-8501, Japan Abstract: The role of deformable liposomes characteristics on skin permeability has evoked considerable interest, since the articles reporting the effectiveness of transfersomes for skin delivery were increasingly published. Several reports focus on the effect of formulation factor which directly affected the transfersome’s skin permeability. However, the effect of formulation factors was not fully understood as the contradictory results. To clarify this problem, the reliable statistical techniques, excellent experimental design and systematical variation were used in this study. Transfersomes loaded meloxicam containing controlled amount of phosphatidylcholine (PC), cholesterol (Chol), type of surfactant (hydrophilic part, lipophilic part ) were prepared and investigated for the physicochemical characteristics (e.g., size, size distribution, charge, elasticity, drug content, morphology) and skin permeability. The results indicated -

Biopharmaceutical Properties of Patches Relevant for Transdermal Drug Absorption – Confounding Factors and In‐Vitro Testing
Biopharmaceutical Properties of Patches 16.06.2015 Relevant for Transdermal Drug Absorption Biopharmaceutical Properties of Patches Relevant for Transdermal Drug Absorption – Confounding Factors and In‐Vitro Testing Johannes Bartholomäus Pharmakreativ Consulting, Aachen Skin as Barrier to Transdermal Absorption And that’s from what Mother Nature did not design all the difficulties in transdermal drug skin as an absorption site. delivery arise. It‘s much more the opposite! Stratum corneum as lipophilic barrier June 16, 2015 J. Bartholomäus: Biopharmaceutical Properties of Patches 2 © Johannes Bartholomäus 1 Biopharmaceutical Properties of Patches 16.06.2015 Relevant for Transdermal Drug Absorption Definition TDDS by Guidelines . Transdermal drug delivery systems . Transdermal patch:** Flexible single‐ (TDDS):* A TDDS or transdermal dose preparation intended to be patch is a flexible pharmaceutical applied to the unbroken skin to preparation of varying size containing obtain a systemic delivery over an one or more active substance(s) to be extended period of time. applied on the intact skin for systemic . Transdermal patches consist of a availability. backing sheet supporting a reservoir . There are two main types of or a matrix containing the active transdermal patch systems substance(s) and on the top a depending on how the drug pressure‐sensitive adhesive, which substance is dispersed in other patch assures the adhesion of the components: preparation to the skin. – Matrix systems with drug release based on . The backing sheet is impermeable to the diffusion of drug substance. the active substance(s) and normally – Reservoir systems containing a specific liquid drug compartment and release is impermeable to water. controlled by a membrane. -

Pulmonary Delivery of Biological Drugs
pharmaceutics Review Pulmonary Delivery of Biological Drugs Wanling Liang 1,*, Harry W. Pan 1 , Driton Vllasaliu 2 and Jenny K. W. Lam 1 1 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong, China; [email protected] (H.W.P.); [email protected] (J.K.W.L.) 2 School of Cancer and Pharmaceutical Sciences, King’s College London, 150 Stamford Street, London SE1 9NH, UK; [email protected] * Correspondence: [email protected]; Tel.: +852-3917-9024 Received: 15 September 2020; Accepted: 20 October 2020; Published: 26 October 2020 Abstract: In the last decade, biological drugs have rapidly proliferated and have now become an important therapeutic modality. This is because of their high potency, high specificity and desirable safety profile. The majority of biological drugs are peptide- and protein-based therapeutics with poor oral bioavailability. They are normally administered by parenteral injection (with a very few exceptions). Pulmonary delivery is an attractive non-invasive alternative route of administration for local and systemic delivery of biologics with immense potential to treat various diseases, including diabetes, cystic fibrosis, respiratory viral infection and asthma, etc. The massive surface area and extensive vascularisation in the lungs enable rapid absorption and fast onset of action. Despite the benefits of pulmonary delivery, development of inhalable biological drug is a challenging task. There are various anatomical, physiological and immunological barriers that affect the therapeutic efficacy of inhaled formulations. This review assesses the characteristics of biological drugs and the barriers to pulmonary drug delivery.