Thermochemistry Review Questions (Chemistry 30)

Thermochemistry Review Questions (Chemistry 30)
- How much heat is required to raise the temperature of 7.35g of water from 21.0°C to 98.0°C?
- How much heat in kJ is required to raise the temperature of 8.00 ounces of water (237g) from 4.0°C to body temperature?
- How much heat in kJ is required to raise the temperature of 2.50g Hg(l) from -2.00°C to 6.00°C. Assume the density of Hg is 13.6g/mL and a molar heat capacity of 28.0J/mol°C.
- When 1.0kg of lead (specific heat capacity 0.160J/g°C) at 100.0°C is added to a quantity of water at 28.5°C, the final temperature of the lead water mixture is 35.2°C. What is the mass of water present?
- A 1.00g copper sample at 100.0°C is added to 50.0g water at 26.5°C. What is the final temperature of the copper water mixture?
- Vanillin is a natural constituent of vanilla. It is also manufactured for use in artificial vanilla flavoring. The combustion of 1.013g of vanillin C8H8O3 in a calorimeter causes 1.17kg of water to heat from 24.89°C to 30.09°C. What is the molar heat of combustion of vanillin?
- The combustion of a 1.176g sample of benzoic acid causes a temperature increase of 4.96°C in a calorimeter containing 1000g of an unknown liquid (not aqueous solution). The heat of combustion of benzoic acid is
-3226kJ/mol. Determine the heat capacity of the liquid in the calorimeter.
- Two solutions, 100.0mL of 1.00mol/L AgNO3(aq) and 100.0mL of 1.00mol/L NaCl(aq) both initially 22.4°C are added to a Styrofoam cup calorimeter and allowed to react. The temperature rises to 30.2°C. Determine the molar enthalpy of the reaction.
- Two solutions, 100.0mL of 1.020mol/L HCl and 50.0mL of 1.988mol/L NaOH, both initially 24.52°C are mixed in a Styrofoam cup calorimeter. Determine the molar enthalpy of neutralization if the final temperature of the mixture is 33.69°C.
- What mass of sucrose must be burned to produce 1.00x103kJ of heat?
- A 25.0mL sample of 0.1045mol/L HCl(g) was neutralized by NaOH(aq). Determine the heat evolved in this neutralization reaction (assume solid and liquid products are only produced)
- The enthalpy of formation for the amino acid leucine C6H13O2N(s) is -637.3kJ/mol. Write the chemical equation to which this value applies. Using this, determine your chemical reaction using only whole number coefficients.
- Calculate the heat of combustion per mole of a gaseous fuel that contains C3H8(g) and C4H10(g).
- Determine the heat of formation of benzene for the following reaction:
2C6H6(l) + 15O2(g) 12CO2(g) + 6H2O(l) ΔH = -6.535x103kJ
- What is the enthalpy change for the reaction of magnesium chloride and sodium hydroxide?
- Use Hess’s law to determine ΔH for the reaction C3H4(g) + 2H2(g) C3H8(g) given that
H2(g) + ½ O2(g) H2O(l)
C3H4(g) + 4O2(g) 3CO3(g) + 2H2O(l) ΔH = -1937kJ
C3H8(l) + 5O2(g) 3CO3(g) + 4H2O(l) ΔH = -2219.1kJ
Electrochemistry Review Questions (Chemistry 30)
- Write the net equation for the redox reaction that occurs in a Scandium Silver voltaic cell (assuming the Scandium Electrode Potential to be -2.02V)
- Draw a silver aluminum voltaic cell. Label the cathode, anode and all other features of the cell. Write the relevant equations under your cell and calculate the cell potential.
- What is the cell potential for the reaction of FeCl2(aq) with Cl2(g)
- Predict the products when Pt electrodes are used in the electrolysis of KI(aq)
- In the electrolysis of AgNO3(aq) what are the expected products if the anode is silver and the cathode is platinum?
- If 12.3g of Cu is deposited at the cathode of an electrolytic cell after 5.50h what was the current used?
- For how long would the electrolysis of copper sulfate have to be carried out using Pt electrodes and a current of 2.13A to produce 2.62L of oxygen gas at 26.2°C and 738mmHg pressure at the anode?
(R = 8.314LkPa/molK = 62.364LmmHg/molK)
- Write the half reactions and the net equation for the following reaction. Label the oxidizing agent / reducing agent, reduction and oxidation half reactions, and calculate the cell potential. Finally, determine whether the reaction is spontaneous or non-spontaneous.
- CuSO4(aq) + Fe(s)
- NaBr + Cl2(g)
- NaCl + Br2(g)
- Fe2(SO4)3(aq) + Al
- Compare and contrast corrosion of iron caused under atmospheric conditions, compared with corrosion under acid rain conditions. Use half reactions in your discussion and list 3 chemical solutions to the problem of corrosion
Thermochemistry Review KEY
- Q = 2.371kJ
- Q = 32.77kJ
- omit…. This question is too in depth for chem 30 (sorry!)
- m = 369.32g
- tf = 26.6348°C
- ΔHm =-3829.0786kJ/mol
- c = 6.26kJ/kg°C
- ΔHm =-6.5364kJ/mol
- ΔHm =-57.98kJ/mol
- m = 66.4g sucrose
- ΔH = Q =0.275kJ
- 6C + 13/2H2 + 1O2 + 1/2N2 C6H13O2N + 637.3kJ
- ΔHcombustion C3H8 = -2043.9kJ/mol, ΔHcombustion C4H10 = -2657.3kJ/mol
- ΔHmf = 49.1kJ/mol
- ΔHrxn = -254kJ
- ΔHrxn =-289.5kJ
Electrochemistry Review KEY
- 3Ag+(aq) + Sc(s) 3Ag(s) + Sc3+(aq)
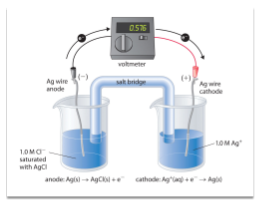 Anode – Oxidation
Anode – Oxidation
Al(s) Al3+(aq) + 3e-
Cathode + Reduction
Ag1+(aq) + 1e- Ag(s)
- E°cell = 0.59V
- I2(g) + H2(g) + 2OH-(aq)
- Silver will plate the platinum electrode
- I = 1.886A
- t = 5.214hours
- a. Fe(s) + Cu2+(aq) Fe2+(aq) + Cu(s) = SPONTANEOUS E°cell = 0.79V
b. Cl2(g) + 2Br1-(aq) 2Cl1-(aq) + Br2(g) = SPOTANEOUS E°cell = 0.29V
c. 2Cl1-(aq) + Br2(g) Cl2(g) + 2Br1-(aq) = NONSPONT E°cell = -0.29V
d. 3Fe3+(aq) + 1Al(s) 3Fe2+(aq) + 1Al3+(aq) = SPONTANEOUS E°cell = 2.43V
- 2Fe(s) + 2H2O(l) + O2(g) 2Fe2+(aq) + 4OH-(aq) regular rusting
2Fe(s) + O2(g) + 4H+(aq) 2Fe2+(aq) + 2H2O(l) acid rusting increases spont
Sacrificial Anode – oxidizes instead of the metal (Fe) bc it’s a stronger RA
Galvanization – coats the metal so nothing can get at it AND is a stronger RA
Painting – coats metal so water, oxygen and acid cannot reach metal
Alloys – mixing a metal with another (i.e. stainless steel)
 Anode – Oxidation
Anode – Oxidation