Exome-Wide Meta-Analysis Identifies Rare 3'-UTR Variant in ERCC1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patients Experiencing Statin-Induced Myalgia Exhibit a Unique Program of Skeletal Muscle Gene Expression Following Statin Re-Challenge
RESEARCH ARTICLE Patients experiencing statin-induced myalgia exhibit a unique program of skeletal muscle gene expression following statin re-challenge Marshall B. Elam1,2,3☯*, Gipsy Majumdar1,2, Khyobeni Mozhui4, Ivan C. Gerling1,3, Santiago R. Vera1, Hannah Fish-Trotter3¤, Robert W. Williams5, Richard D. Childress1,3, Rajendra Raghow1,2☯* 1 Department of Veterans Affairs Medical Center-Memphis, Memphis, Tennessee, United States of America, 2 Department of Pharmacology, University of Tennessee Health Sciences Center, Memphis, Tennessee, a1111111111 United States of America, 3 Department of Medicine, University of Tennessee Health Sciences Center, a1111111111 Memphis, Tennessee, United States of America, 4 Department of Preventive Medicine, University of a1111111111 Tennessee Health Sciences Center, Memphis, Tennessee, United States of America, 5 Department of a1111111111 Genetics, Genomics and Informatics, College of Medicine, University of Tennessee Health Science Center, a1111111111 Memphis, Tennessee, United States of America ☯ These authors contributed equally to this work. ¤ Current address: Department of Rehabilitation Medicine, Vanderbilt University, Nashville, Tennessee, United States of America. * [email protected] (MBE); [email protected] (RR) OPEN ACCESS Citation: Elam MB, Majumdar G, Mozhui K, Gerling IC, Vera SR, Fish-Trotter H, et al. (2017) Patients Abstract experiencing statin-induced myalgia exhibit a unique program of skeletal muscle gene Statins, the 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase inhibitors, are widely pre- expression following statin re-challenge. PLoS ONE scribed for treatment of hypercholesterolemia. Although statins are generally well tolerated, 12(8): e0181308. https://doi.org/10.1371/journal. pone.0181308 up to ten percent of statin-treated patients experience myalgia symptoms, defined as mus- cle pain without elevated creatinine phosphokinase (CPK) levels. -

Location Analysis of Estrogen Receptor Target Promoters Reveals That
Location analysis of estrogen receptor ␣ target promoters reveals that FOXA1 defines a domain of the estrogen response Jose´ e Laganie` re*†, Genevie` ve Deblois*, Ce´ line Lefebvre*, Alain R. Bataille‡, Franc¸ois Robert‡, and Vincent Gigue` re*†§ *Molecular Oncology Group, Departments of Medicine and Oncology, McGill University Health Centre, Montreal, QC, Canada H3A 1A1; †Department of Biochemistry, McGill University, Montreal, QC, Canada H3G 1Y6; and ‡Laboratory of Chromatin and Genomic Expression, Institut de Recherches Cliniques de Montre´al, Montreal, QC, Canada H2W 1R7 Communicated by Ronald M. Evans, The Salk Institute for Biological Studies, La Jolla, CA, July 1, 2005 (received for review June 3, 2005) Nuclear receptors can activate diverse biological pathways within general absence of large scale functional data linking these putative a target cell in response to their cognate ligands, but how this binding sites with gene expression in specific cell types. compartmentalization is achieved at the level of gene regulation is Recently, chromatin immunoprecipitation (ChIP) has been used poorly understood. We used a genome-wide analysis of promoter in combination with promoter or genomic DNA microarrays to occupancy by the estrogen receptor ␣ (ER␣) in MCF-7 cells to identify loci recognized by transcription factors in a genome-wide investigate the molecular mechanisms underlying the action of manner in mammalian cells (20–24). This technology, termed 17-estradiol (E2) in controlling the growth of breast cancer cells. ChIP-on-chip or location analysis, can therefore be used to deter- We identified 153 promoters bound by ER␣ in the presence of E2. mine the global gene expression program that characterize the Motif-finding algorithms demonstrated that the estrogen re- action of a nuclear receptor in response to its natural ligand. -

Exome-Wide Meta-Analysis Identifies Rare 3'-UTR Variant In
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Erasmus University Digital Repository ORIGINAL RESEARCH published: 18 October 2017 doi: 10.3389/fgene.2017.00151 Exome-Wide Meta-Analysis Identifies Rare 3′-UTR Variant in ERCC1/CD3EAP Associated with Symptoms of Sleep Apnea Ashley van der Spek 1, Annemarie I. Luik 2, Desana Kocevska 3, Chunyu Liu 4, 5, 6, Rutger W. W. Brouwer 7, Jeroen G. J. van Rooij 8, 9, 10, Mirjam C. G. N. van den Hout 7, Robert Kraaij 1, 8, 9, Albert Hofman 1, 11, André G. Uitterlinden 1, 8, 9, Wilfred F. J. van IJcken 7, Daniel J. Gottlieb 12, 13, 14, Henning Tiemeier 1, 15, Cornelia M. van Duijn 1 and Najaf Amin 1* 1 Department of Epidemiology, Erasmus Medical Center, Rotterdam, Netherlands, 2 Sleep and Circadian Neuroscience Institute, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 3 Department of Child and Adolescent Psychiatry, Erasmus Medical Center, Rotterdam, Netherlands, 4 Framingham Heart Study, National Heart, Lung, and Blood Institute, Framingham, MA, United States, 5 Population Sciences Branch, National Heart, Lung, and Blood Institute, Bethesda, MD, United States, 6 Department of Biostatistics, School of Public Health, Boston University, Boston, MA, United States, 7 Center for Biomics, Erasmus Medical Center, Rotterdam, Netherlands, 8 Department of Internal Medicine, Erasmus Medical Center, Rotterdam, Netherlands, 9 Netherlands Consortium for Healthy Ageing, Rotterdam, Netherlands, 10 Department of Neurology, Erasmus -

The Capacity of Long-Term in Vitro Proliferation of Acute Myeloid
The Capacity of Long-Term in Vitro Proliferation of Acute Myeloid Leukemia Cells Supported Only by Exogenous Cytokines Is Associated with a Patient Subset with Adverse Outcome Annette K. Brenner, Elise Aasebø, Maria Hernandez-Valladares, Frode Selheim, Frode Berven, Ida-Sofie Grønningsæter, Sushma Bartaula-Brevik and Øystein Bruserud Supplementary Material S2 of S31 Table S1. Detailed information about the 68 AML patients included in the study. # of blasts Viability Proliferation Cytokine Viable cells Change in ID Gender Age Etiology FAB Cytogenetics Mutations CD34 Colonies (109/L) (%) 48 h (cpm) secretion (106) 5 weeks phenotype 1 M 42 de novo 241 M2 normal Flt3 pos 31.0 3848 low 0.24 7 yes 2 M 82 MF 12.4 M2 t(9;22) wt pos 81.6 74,686 low 1.43 969 yes 3 F 49 CML/relapse 149 M2 complex n.d. pos 26.2 3472 low 0.08 n.d. no 4 M 33 de novo 62.0 M2 normal wt pos 67.5 6206 low 0.08 6.5 no 5 M 71 relapse 91.0 M4 normal NPM1 pos 63.5 21,331 low 0.17 n.d. yes 6 M 83 de novo 109 M1 n.d. wt pos 19.1 8764 low 1.65 693 no 7 F 77 MDS 26.4 M1 normal wt pos 89.4 53,799 high 3.43 2746 no 8 M 46 de novo 26.9 M1 normal NPM1 n.d. n.d. 3472 low 1.56 n.d. no 9 M 68 MF 50.8 M4 normal D835 pos 69.4 1640 low 0.08 n.d. -

Fine Mapping Studies of Quantitative Trait Loci for Baseline Platelet Count in Mice and Humans
Fine mapping studies of quantitative trait loci for baseline platelet count in mice and humans A thesis submitted in fulfillment of the requirements for the degree of Doctor of Philosophy Melody C Caramins December 2010 University of New South Wales ORIGINALITY STATEMENT ‘I hereby declare that this submission is my own work and to the best of my knowledge it contains no materials previously published or written by another person, or substantial proportions of material which have been accepted for the award of any other degree or diploma at UNSW or any other educational institution, except where due acknowledgement is made in the thesis. Any contribution made to the research by others, with whom I have worked at UNSW or elsewhere, is explicitly acknowledged in the thesis. I also declare that the intellectual content of this thesis is the product of my own work, except to the extent that assistance from others in the project's design and conception or in style, presentation and linguistic expression is acknowledged.’ Signed …………………………………………….............. Date …………………………………………….............. This thesis is dedicated to my father. Dad, thanks for the genes – and the environment! ACKNOWLEDGEMENTS “Nothing can come out of nothing, any more than a thing can go back to nothing.” - Marcus Aurelius Antoninus A PhD thesis is never the work of one person in isolation from the world at large. I would like to thank the following people, without whom this work would not have existed. Thank you firstly, to all my teachers, of which there have been many. Undoubtedly, the greatest debt is owed to my supervisor, Dr Michael Buckley. -

Noelia Díaz Blanco
Effects of environmental factors on the gonadal transcriptome of European sea bass (Dicentrarchus labrax), juvenile growth and sex ratios Noelia Díaz Blanco Ph.D. thesis 2014 Submitted in partial fulfillment of the requirements for the Ph.D. degree from the Universitat Pompeu Fabra (UPF). This work has been carried out at the Group of Biology of Reproduction (GBR), at the Department of Renewable Marine Resources of the Institute of Marine Sciences (ICM-CSIC). Thesis supervisor: Dr. Francesc Piferrer Professor d’Investigació Institut de Ciències del Mar (ICM-CSIC) i ii A mis padres A Xavi iii iv Acknowledgements This thesis has been made possible by the support of many people who in one way or another, many times unknowingly, gave me the strength to overcome this "long and winding road". First of all, I would like to thank my supervisor, Dr. Francesc Piferrer, for his patience, guidance and wise advice throughout all this Ph.D. experience. But above all, for the trust he placed on me almost seven years ago when he offered me the opportunity to be part of his team. Thanks also for teaching me how to question always everything, for sharing with me your enthusiasm for science and for giving me the opportunity of learning from you by participating in many projects, collaborations and scientific meetings. I am also thankful to my colleagues (former and present Group of Biology of Reproduction members) for your support and encouragement throughout this journey. To the “exGBRs”, thanks for helping me with my first steps into this world. Working as an undergrad with you Dr. -

Core Circadian Clock Transcription Factor BMAL1 Regulates Mammary Epithelial Cell
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.23.432439; this version posted February 23, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Title: Core circadian clock transcription factor BMAL1 regulates mammary epithelial cell 2 growth, differentiation, and milk component synthesis. 3 Authors: Theresa Casey1ǂ, Aridany Suarez-Trujillo1, Shelby Cummings1, Katelyn Huff1, 4 Jennifer Crodian1, Ketaki Bhide2, Clare Aduwari1, Kelsey Teeple1, Avi Shamay3, Sameer J. 5 Mabjeesh4, Phillip San Miguel5, Jyothi Thimmapuram2, and Karen Plaut1 6 Affiliations: 1. Department of Animal Science, Purdue University, West Lafayette, IN, USA; 2. 7 Bioinformatics Core, Purdue University; 3. Animal Science Institute, Agriculture Research 8 Origination, The Volcani Center, Rishon Letsiyon, Israel. 4. Department of Animal Sciences, 9 The Robert H. Smith Faculty of Agriculture, Food, and Environment, The Hebrew University of 10 Jerusalem, Rehovot, Israel. 5. Genomics Core, Purdue University 11 Grant support: Binational Agricultural Research Development (BARD) Research Project US- 12 4715-14; Photoperiod effects on milk production in goats: Are they mediated by the molecular 13 clock in the mammary gland? 14 ǂAddress for correspondence. 15 Theresa M. Casey 16 BCHM Room 326 17 175 South University St. 18 West Lafayette, IN 47907 19 Email: [email protected] 20 Phone: 802-373-1319 21 22 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.23.432439; this version posted February 23, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Tissue-Specific Transcriptome for Poeciliopsis Prolifica Reveals
INVESTIGATION Tissue-Specific Transcriptome for Poeciliopsis prolifica Reveals Evidence for Genetic Adaptation Related to the Evolution of a Placental Fish Nathaniel K. Jue,*,1 Robert J. Foley,* David N. Reznick,† Rachel J. O’Neill,* and Michael J. O’Neill*,2 *Institute for Systems Genomics and Department of Molecular and Cell Biology, University of Connecticut, Storrs, CT 06269 and †Department of Biology, University of California, Riverside, CA 92521 ABSTRACT The evolution of the placenta is an excellent model to examine the evolutionary processes KEYWORDS underlying adaptive complexity due to the recent, independent derivation of placentation in divergent transcriptome animal lineages. In fishes, the family Poeciliidae offers the opportunity to study placental evolution with positive selection respect to variation in degree of post-fertilization maternal provisioning among closely related sister gene expression species. In this study, we present a detailed examination of a new reference transcriptome sequence for the placenta live-bearing, matrotrophic fish, Poeciliopsis prolifica, from multiple-tissue RNA-seq data. We describe the fish genetic components active in liver, brain, late-stage embryo, and the maternal placental/ovarian complex, as well as associated patterns of positive selection in a suite of orthologous genes found in fishes. Results indicate the expression of many signaling transcripts, “non-coding” sequences and repetitive elements in the maternal placental/ovarian complex. Moreover, patterns of positive selection in protein sequence evolution were found associated with live-bearing fishes, generally, and the placental P. prolifica, specifi- cally, that appear independent of the general live-bearer lifestyle. Much of the observed patterns of gene expression and positive selection are congruent with the evolution of placentation in fish functionally converging with mammalian placental evolution and with the patterns of rapid evolution facilitated by the teleost-specific whole genome duplication event. -

Chromosome 20 Shows Linkage with DSM-IV Nicotine Dependence in Finnish Adult Smokers
Nicotine & Tobacco Research, Volume 14, Number 2 (February 2012) 153–160 Original Investigation Chromosome 20 Shows Linkage With DSM-IV Nicotine Dependence in Finnish Adult Smokers Kaisu Keskitalo-Vuokko, Ph.D.,1 Jenni Hällfors, M.Sc.,1,2 Ulla Broms, Ph.D.,1,3 Michele L. Pergadia, Ph.D.,4 Scott F. Saccone, Ph.D.,4 Anu Loukola, Ph.D.,1,3 Pamela A. F. Madden, Ph.D.,4 & Jaakko Kaprio, M.D., Ph.D.1,2,3 1 Hjelt Institute, Department of Public Health, University of Helsinki, Helsinki, Finland 2 Institute for Molecular Medicine Finland (FIMM), University of Helsinki, Helsinki, Finland 3 National Institute for Health and Welfare (THL), Helsinki, Finland 4 Department of Psychiatry, Washington University School of Medicine, St. Louis, MO Corresponding Author: Jaakko Kaprio, M.D., Ph.D., Department of Public Health, University of Helsinki, PO Box 41 (Manner- heimintie 172), Helsinki 00014, Finland. Telephone: +358-9-191-27595; Fax: +358-9-19127570; E-mail: [email protected] Received February 5, 2011; accepted June 21, 2011 2009). Despite several gene-mapping studies, the genes underlying Abstract liability to nicotine dependence (ND) remain largely unknown. Introduction: Chromosome 20 has previously been associated Recently, Han, Gelernter, Luo, and Yang (2010) performed a with nicotine dependence (ND) and smoking cessation. Our meta-analysis of 15 genome-wide linkage scans of smoking aim was to replicate and extend these findings. behavior. Linkage signals were observed on chromosomal regions 17q24.3–q25.3, 5q33.1–q35.2, 20q13.12–32, and 22q12.3–13.32. Methods: First, a total of 759 subjects belonging to 206 Finnish The relevance of the chromosome 20 finding is highlighted families were genotyped with 18 microsatellite markers residing by the fact that CHRNA4 encoding the nicotinic acetylcholine on chromosome 20, in order to replicate previous linkage findings. -

A Novel PLEKHA7 Interactor at Adherens Junctions
Thesis PDZD11: a novel PLEKHA7 interactor at adherens junctions GUERRERA, Diego Abstract PLEKHA7 is a recently identified protein of the AJ that has been involved by genetic and genomic studies in the regulation of miRNA signaling and cardiac contractility, hypertension and glaucoma. However, the molecular mechanisms behind PLEKHA7 involvement in tissue physiology and pathology remain unknown. In my thesis I report novel results which uncover PLEKHA7 functions in epithelial and endothelial cells, through the identification of a novel molecular interactor of PLEKHA7, PDZD11, by yeast two-hybrid screening, mass spectrometry, co-immunoprecipitation and pulldown assays. I dissected the structural basis of their interaction, showing that the WW domain of PLEKHA7 binds to the N-terminal region of PDZD11; this interaction mediates the junctional recruitment of PDZD11, identifying PDZD11 as a novel AJ protein. I provided evidence that PDZD11 forms a complex with nectins at AJ, its PDZ domain binds to the PDZ-binding motif of nectins. PDZD11 stabilizes nectins promoting the early steps of junction assembly. Reference GUERRERA, Diego. PDZD11: a novel PLEKHA7 interactor at adherens junctions. Thèse de doctorat : Univ. Genève, 2016, no. Sc. 4962 URN : urn:nbn:ch:unige-877543 DOI : 10.13097/archive-ouverte/unige:87754 Available at: http://archive-ouverte.unige.ch/unige:87754 Disclaimer: layout of this document may differ from the published version. 1 / 1 UNIVERSITE DE GENÈVE FACULTE DES SCIENCES Section de Biologie Prof. Sandra Citi Département de Biologie Cellulaire PDZD11: a novel PLEKHA7 interactor at adherens junctions THÈSE Présentée à la Faculté des sciences de l’Université de Genève Pour obtenir le grade de Doctor ès science, mention Biologie par DIEGO GUERRERA de Benevento (Italie) Thèse N° 4962 GENÈVE Atelier d'impression Repromail 2016 1 Table of contents RÉSUMÉ .................................................................................................................. -

Chromosomal Microarray Analysis in Turkish Patients with Unexplained Developmental Delay and Intellectual Developmental Disorders
177 Arch Neuropsychitry 2020;57:177−191 RESEARCH ARTICLE https://doi.org/10.29399/npa.24890 Chromosomal Microarray Analysis in Turkish Patients with Unexplained Developmental Delay and Intellectual Developmental Disorders Hakan GÜRKAN1 , Emine İkbal ATLI1 , Engin ATLI1 , Leyla BOZATLI2 , Mengühan ARAZ ALTAY2 , Sinem YALÇINTEPE1 , Yasemin ÖZEN1 , Damla EKER1 , Çisem AKURUT1 , Selma DEMİR1 , Işık GÖRKER2 1Faculty of Medicine, Department of Medical Genetics, Edirne, Trakya University, Edirne, Turkey 2Faculty of Medicine, Department of Child and Adolescent Psychiatry, Trakya University, Edirne, Turkey ABSTRACT Introduction: Aneuploids, copy number variations (CNVs), and single in 39 (39/123=31.7%) patients. Twelve CNV variant of unknown nucleotide variants in specific genes are the main genetic causes of significance (VUS) (9.75%) patients and 7 CNV benign (5.69%) patients developmental delay (DD) and intellectual disability disorder (IDD). were reported. In 6 patients, one or more pathogenic CNVs were These genetic changes can be detected using chromosome analysis, determined. Therefore, the diagnostic efficiency of CMA was found to chromosomal microarray (CMA), and next-generation DNA sequencing be 31.7% (39/123). techniques. Therefore; In this study, we aimed to investigate the Conclusion: Today, genetic analysis is still not part of the routine in the importance of CMA in determining the genomic etiology of unexplained evaluation of IDD patients who present to psychiatry clinics. A genetic DD and IDD in 123 patients. diagnosis from CMA can eliminate genetic question marks and thus Method: For 123 patients, chromosome analysis, DNA fragment analysis alter the clinical management of patients. Approximately one-third and microarray were performed. Conventional G-band karyotype of the positive CMA findings are clinically intervenable. -
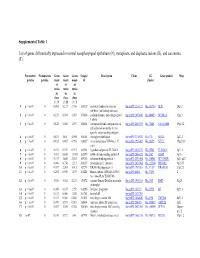
Supplemental Table 1 List of Genes Differentially Expressed In
Supplemental Table 1 List of genes differentially expressed in normal nasopharyngeal epithelium (N), metaplastic and displastic lesions (R), and carcinoma (T). Parametric Permutation Geom Geom Geom Unique Description Clone UG Gene symbol Map p-value p-value mean mean mean id cluster of of of ratios ratios ratios in in in class class class 1 : N 2 : R 3 : T 1 p < 1e-07 0 0.061 0.123 2.708 169329 secretory leukocyte protease IncytePD:2510171 Hs.251754 SLPI 20q12 inhibitor (antileukoproteinase) 2 p < 1e-07 0 0.125 0.394 1.863 163628 sodium channel, nonvoltage-gated IncytePD:1453049 Hs.446415 SCNN1A 12p13 1 alpha 3 p < 1e-07 0 0.122 0.046 1.497 160401 carcinoembryonic antigen-related IncytePD:2060355 Hs.73848 CEACAM6 19q13.2 cell adhesion molecule 6 (non- specific cross reacting antigen) 4 p < 1e-07 0 0.675 1.64 5.594 165101 monoglyceride lipase IncytePD:2174920 Hs.6721 MGLL 3q21.3 5 p < 1e-07 0 0.182 0.487 0.998 166827 nei endonuclease VIII-like 1 (E. IncytePD:1926409 Hs.28355 NEIL1 15q22.33 coli) 6 p < 1e-07 0 0.194 0.339 0.915 162931 hypothetical protein FLJ22418 IncytePD:2816379 Hs.36563 FLJ22418 1p11.1 7 p < 1e-07 0 1.313 0.645 13.593 162399 S100 calcium binding protein P IncytePD:2060823 Hs.2962 S100P 4p16 8 p < 1e-07 0 0.157 1.445 2.563 169315 selenium binding protein 1 IncytePD:2591494 Hs.334841 SELENBP1 1q21-q22 9 p < 1e-07 0 0.046 0.738 1.213 160115 prominin-like 1 (mouse) IncytePD:2070568 Hs.112360 PROML1 4p15.33 10 p < 1e-07 0 0.787 2.264 3.013 167294 HRAS-like suppressor 3 IncytePD:1969263 Hs.37189 HRASLS3 11q12.3 11 p < 1e-07 0 0.292 0.539 1.493 168221 Homo sapiens cDNA FLJ13510 IncytePD:64451 Hs.37896 2 fis, clone PLACE1005146.