Partial Monosomy14q Involving FOXG1 and NOVA1 in an Infant with Microcephaly, Seizures and Severe Developmental Delay H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retted Q&A: Epilepsy Incidence, Treatments and Research Dr. Eric
RettEd Q&A: Epilepsy Incidence, Treatments and Research Dr. Eric Marsh, MD PhD, Medical Director Rett Clinic, NHS Investigator, and Basic Scientist, Children’s Hospital of Philadelphia Webcast 09/11/2018 Facilitator: Paige Nues, Rettsyndrome.org Recording link: https://attendee.gotowebinar.com/recording/2303318923907771398 ATTENDEE QUESTIONS RESPONSE REFERENCES AED Questions: Treatments and Triggers I want to know what about relation between Stressors are anecdotally reported to lower the seizure pain, digestive pain, apnea and seizure threshold- these would all be stressful features that could discharge. lower seizure threshold and theoretically increase seizure frequency. Any opinions on fycompa? Very limited experience so far to make a clear judgment. I have tried it in 2 Rett patients without great success, both in patients with very severe seizures. Is there a way to tell the difference between There is a slide in the talk that goes over some features, such a seizure and a “Rett spell” other than EEG? as retained awareness, repetitive nature of the events, that can give some hints as to the nature of the event, but only EEG can definitively discern the difference. My daughter has epilepsy and always has Without knowing all of her clinical details, it is hard for me to seizures. She takes medication Onfi and give recommendations. Depending on seizure types, EEG, Keppra and still have seizures. What should and previous medications tried, I would suggest other meds I do? I need help and ideas thanks. such as Rufinamide, valproic acid or trying the ketogenic diet. Do you see seizures connected to puberty or You can look at Jane Lane’s RettEd about puberty. -

Communication Intervention in Rett Syndrome: a Systematic Review Research in Autism Spectrum Disorders
Research in Autism Spectrum Disorders 3 (2009) 304–318 Contents lists available at ScienceDirect Research in Autism Spectrum Disorders Journal homepage: http://ees.elsevier.com/RASD/default.asp Review Communication intervention in Rett syndrome: A systematic review Jeff Sigafoos a,*, Vanessa A. Green a, Ralf Schlosser b, Mark F. O’eilly c, Giulio E. Lancioni d, Mandy Rispoli c, Russell Lang c a Victoria University of Wellington, New Zealand b Northeastern University, Boston and Childrens Hospital Boston at Waltham, MA, USA c Meadows Center for Preventing Educational Risk, The University of Texas at Austin, Austin, TX, USA d University of Bari, Bari, Italy ARTICLE INFO ABSTRACT Article history: We reviewed communication intervention studies involving people Received 25 September 2008 with Rett syndrome. Systematic searches of five electronic databases, Accepted 26 September 2008 selected journals, and reference lists identified nine studies meeting the inclusion criteria. These studies were evaluated in terms of: (a) Keywords: participant characteristics, (b) target skills, (c) procedures, (d) main Communication intervention findings, and (e) certainty of evidence. Across the nine studies, Rett syndrome intervention was provided to a total of 31 participants aged 2:7–17:0 Systematic review (years:months). Communication modes included speech, gestures, communication boards, and computer-based systems. Targeted communication functions included imitative speech, requesting, naming/commenting, and various receptive language skills (e.g., respond to requests, answer questions, receptively identify symbols). Intervention approaches included early intensive behavioral inter- vention, systematic instruction, and music therapy. Positive out- comes were reported for 26 (84%) of the 31 participants. However, these outcomes must be interpreted with caution because the certainty of evidence was inconclusive for all but one of the studies. -

Epilepsy and Rett Syndrome
EPILEPSY AND RETT SYNDROME Liisa Metsähonkala, Pediatric Neurologist Epilepsia-Helsinki, Helsinki University Hospital HUS Helsinki University Hospital RETT Epilepsy -syndrome Epilepsy in Epilepsy in Rett Rett syndrome syndrome Epilepsy may be a major or minor problem for people with Rett syndrome and their families 2 28.9.19 HUS Helsinki University Hospital EPILEPSY AND RETT SYNDROME - Some general aspects of epilepsy - Epilepsy in Rett syndrome (focus on children) - Diversity of epileptic seizures - Diversity of nonepileptic paroxysmal attacks - How to make the diagnosis? - Treatment of epilepsy 3 28.9.19 HUS Helsinki University Hospital WHAT IS EPILEPSY ? International - epilepsy is not one disease but a large group of different League disorders Against Epilepsy - an epileptic seizure is a transient occurrence of signs and/ or symptoms due to abnormal and certain kind of neuronal activity (excessive or synchronous) in the brain - versus nonepileptic symptoms - epilepsy is a disease characterized by an enduring predisposition to generate epileptic seizures – versus seizures that anyone can have because of an acute provoking factor 4 28.9.19 HUS Helsinki University Hospital Seizure types Etiology Focal Generalized Unknown onset onset onset Structural Genetic Epilepsy types Infectious Combined FocalFocal Generalized Unknown Generalized Metabolic & Focal Immune Co-morbidities Unknown Epilepsy Syndromes HUS Helsinki University Hospital Generalized seizures Focal seizures • Originate at some point within • Originate within networks and rapidly -

Whole-Genome Sequencing Reveals Principles of Brain Retrotransposition in Neurodevelopmental Disorders
Cell Research (2018) 28:187-203. © 2018 IBCB, SIBS, CAS All rights reserved 1001-0602/18 $ 32.00 ORIGINAL ARTICLE www.nature.com/cr Whole-genome sequencing reveals principles of brain retrotransposition in neurodevelopmental disorders Jasmine Jacob-Hirsch1, 2, Eran Eyal1, Binyamin A Knisbacher2, Jonathan Roth3, 5, Karen Cesarkas1, Chen Dor1, Sarit Farage-Barhom1, Vered Kunik1, Amos J Simon1, Moran Gal2, Michal Yalon4, Sharon Moshitch-Moshkovitz1, Rick Tearle6, Shlomi Constantini3, 5, Erez Y Levanon2, Ninette Amariglio1, 2, Gideon Rechavi1, 5 1Cancer Research Center and the Wohl Institute of Translational Medicine, the Chaim Sheba Medical Center, Tel Hashomer, Is- rael; 2Mina and Everard Goodman Faculty of Life Sciences, Bar Ilan University, Israel; 3Department of Pediatric Neurosurgery, Dana Children’s Hospital, Tel Aviv Medical Center, Israel; 4Department of Pediatric Hematology-Oncology, Edmond and Lily Sa- fra Children’s Hospital, The Chaim Sheba Medical Center, Tel Hashomer, Israel; 5Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; 6Complete Genomics, 2071 Stierlin Court, Mountain View, CA 94043, USA Neural progenitor cells undergo somatic retrotransposition events, mainly involving L1 elements, which can be potentially deleterious. Here, we analyze the whole genomes of 20 brain samples and 80 non-brain samples, and char- acterized the retrotransposition landscape of patients affected by a variety of neurodevelopmental disorders including Rett syndrome, tuberous sclerosis, ataxia-telangiectasia and autism. We report that the number of retrotranspositions in brain tissues is higher than that observed in non-brain samples and even higher in pathologic vs normal brains. The majority of somatic brain retrotransposons integrate into pre-existing repetitive elements, preferentially A/T rich L1 sequences, resulting in nested insertions. -

Epilepsy & Behavior
Epilepsy & Behavior 66 (2017) 27–33 Contents lists available at ScienceDirect Epilepsy & Behavior journal homepage: www.elsevier.com/locate/yebeh Effectiveness and tolerability of antiepileptic drugs in 104 girls with Rett syndrome Aglaia Vignoli, Miriam Nella Savini ⁎, Maria Sonia Nowbut, Angela Peron, Katherine Turner, Francesca La Briola, Maria Paola Canevini Epilepsy Center, San Paolo Hospital, Department of Health Sciences, Università degli Studi di Milano, Italy article info abstract Article history: Approximately 60–80% of girls with Rett Syndrome (RTT) have epilepsy, which represents one of the most severe Received 29 July 2016 problems clinicians have to deal with, especially when patients are 7–12 years old. Revised 6 October 2016 The aim of this study was to analyze the antiepileptic drugs (AEDs) prescribed in RTT, and to assess their effec- Accepted 8 October 2016 tiveness and tolerability in different age groups from early infancy to adulthood. Available online xxxx We included in this study 104 girls, aged 2–42 years (mean age 13.9 years): 89 had a mutation in MECP2,5in Keywords: CDKL5,2inFOXG1, and the mutational status was unknown in the remaining 8. Rett syndrome Epilepsy was present in 82 patients (79%). Mean age at epilepsy onset was 4.1 years. Epilepsy We divided the girls into 5 groups according to age: b5, 5–9, 10–14, 15–19, 20 years and older. Antiepileptic drugs Valproic acid (VPA) was the most prescribed single therapy in young patients (b15 years), whereas carbamaze- Age-dependency pine (CBZ) was preferred by clinicians in older patients. The most frequently adopted AED combination in the pa- tients younger than 10 years and older than 15 was VPA and lamotrigine (LTG). -

Evidence-Based Physical Therapy for Individuals with Rett Syndrome: a Systematic Review
brain sciences Review Evidence-Based Physical Therapy for Individuals with Rett Syndrome: A Systematic Review Marta Fonzo, Felice Sirico and Bruno Corrado * Department of Public Health, University of Naples “Federico II”, 80131 Naples, Italy; [email protected] (M.F.); [email protected] (F.S.) * Correspondence: [email protected]; Tel.: +39-081-7462795 Received: 14 June 2020; Accepted: 29 June 2020; Published: 30 June 2020 Abstract: Rett syndrome is a rare genetic disorder that affects brain development and causes severe mental and physical disability. This systematic review analyzes the most recent evidence concerning the role of physical therapy in the management of individuals with Rett syndrome. The review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. A total of 17319 studies were found in the main scientific databases. Applying the inclusion/exclusion criteria, 22 studies were admitted to the final phase of the review. Level of evidence of the included studies was assessed using the Oxford Centre for Evidence-Based Medicine—Levels of Evidence guide. Nine approaches to physical therapy for patients with Rett syndrome were identified: applied behavior analysis, conductive education, environmental enrichment, traditional physiotherapy with or without aids, hydrotherapy, treadmill, music therapy, computerized systems, and sensory-based treatment. It has been reported that patients had clinically benefited from the analysed approaches despite the fact that they did not have strong research evidence. According to the results, a multimodal individualized physical therapy program should be regularly recommended to patients with Rett syndrome in order to preserve autonomy and to improve quality of life. -

The ICD-10 Classification of Mental and Behavioural Disorders : Clinical Descriptions and Diagnostic Guidelines
ICD-10 ThelCD-10 Classification of Mental and Behavioural Disorders Clinical descriptions and diagnostic guidelines | World Health Organization I Geneva I 1992 Reprinted 1993, 1994, 1995, 1998, 2000, 2002, 2004 WHO Library Cataloguing in Publication Data The ICD-10 classification of mental and behavioural disorders : clinical descriptions and diagnostic guidelines. 1.Mental disorders — classification 2.Mental disorders — diagnosis ISBN 92 4 154422 8 (NLM Classification: WM 15) © World Health Organization 1992 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers' products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Diagnosis Questions
Diagnosis questions What is Rett Syndrome and what causes it? Rett Syndrome is a neurological or brain disorder which most often affects apparently healthy little girls around the age of 6-18 months. Early signs are autism like behaviours with the loss of speech and hand use. Your daughter might stop playing or interacting normally. Onset can be sudden or more subtle, where you can't quite remember the last time she waved or said a word. She may have unusual hand movements when she is awake; in Rett S yndrome, hands are usually clasped or held together in the middle of her body (midline) or she may wring them together or move them from hand to mouth. She may have other problems, such as strange breathing patterns, screaming episodes or sleep disturbances. Rett Syndrome is named after Austrian doctor, Andreas Rett, who first identified the condition in 1966. It is as common as Huntington's Disease, although not very well known and occurs in 1:10,000 live female births. The condition is most often caused by mutations or faults in the gene MECP2. This gene is on the X chromosome which is why the condition most often affects girls. There are four stages of Rett Syndrome. The condition is not degenerative as it was once thought to be. Degenerative means that there is progressive deterioration of nerve cells, leading to cell death; this does not occur in Rett Syndrome. The condition does however, usually follow a path of progression, where skills such as speech, hand use and mobility are lost. -

A Post-Transcriptional Mechanism Pacing Expression of Neural Genes with Precursor Cell Differentiation Status
ARTICLE Received 24 Sep 2014 | Accepted 21 May 2015 | Published 6 Jul 2015 DOI: 10.1038/ncomms8576 OPEN A post-transcriptional mechanism pacing expression of neural genes with precursor cell differentiation status Weijun Dai1, Wencheng Li2, Mainul Hoque2, Zhuyun Li1, Bin Tian2 & Eugene V. Makeyev1,3 Nervous system (NS) development relies on coherent upregulation of extensive sets of genes in a precise spatiotemporal manner. How such transcriptome-wide effects are orchestrated at the molecular level remains an open question. Here we show that 30-untranslated regions (30 UTRs) of multiple neural transcripts contain AU-rich cis-elements (AREs) recognized by tristetraprolin (TTP/Zfp36), an RNA-binding protein previously implicated in regulation of mRNA stability. We further demonstrate that the efficiency of ARE-dependent mRNA degradation declines in the neural lineage because of a decrease in the TTP protein expression mediated by the NS-enriched microRNA miR-9. Importantly, TTP downregulation in this context is essential for proper neuronal differentiation. On the other hand, inactivation of TTP in non-neuronal cells leads to dramatic upregulation of multiple NS-specific genes. We conclude that the newly identified miR-9/TTP circuitry limits unscheduled accumulation of neuronal mRNAs in non-neuronal cells and ensures coordinated upregulation of these transcripts in neurons. 1 School of Biological Sciences, Nanyang Technological University, Singapore 637551, Singapore. 2 Department of Microbiology, Biochemistry, and Molecular Genetics, Rutgers New Jersey Medical School, Newark, New Jersey 07103, USA. 3 MRC Centre for Developmental Neurobiology, King’s College London, London SE1 1UL, UK. Correspondence and requests for materials should be addressed to E.V.M. -

Physician Guide for Autism Spectrum Disorders
Jonah | age 8 | Riverview,Rive rvie w, MI Physician Guide for Autism Spectrum Disorders Created by 1 Derek | age 5 | Canton, MI AAoM Mission OUR MISSION The mission of Autism Alliance of Michigan is to lead collaborative efforts across the state that will improve the quality of life for individuals with autism through education, comprehensive services, community awareness, inclusion efforts, and coordinated advocacy. HOW WE INVEST IN THE MISSION The Autism Alliance of Michigan (AAoM) is leading efforts to make Michigan a better place to live for people with autism and their families. Our impact on families, communities, lawmakers, and service providers is not possible without the generous support of our corporate, foundation, and individual donors. 2 Physician Guide for Autism Spectrum Disorder Autism Screening • Research has found that Autism Spectrum Disorder (ASD) can be detected as early as 18 months or younger. By age 2, a diagnosis by an experienced clinician can be considered very reliable. However, many children do not receive a diagnosis until they are much older. This delay means children with ASD may not get the help that they need during their early years, which has been deemed so effective. The earlier ASD is diagnosed, the earlier treatment can begin. Screening tools are designed to help identify children who might have developmental delays. Screening tools do not provide conclusive evidence and do not result in a diagnosis. A positive developmental screening result for autism should be followed up with a referral to a developmental specialist, psychologist or autism evaluation center. Types of Screening Tools The American Academy of Pediatrics (AAP) recommends that all children receive autism-specific screening at 18 and 24 months of age, in addition to broad developmental screening at 9, 18, and 24 months. -
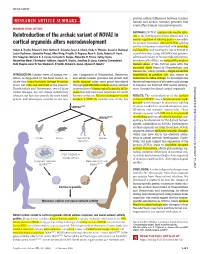
Reintroduction of the Archaic Variant of NOVA1
RESEARCH ◥ protein-coding differences between modern RESEARCH ARTICLE SUMMARY human and archaic hominin genomes that could affect human neurodevelopment. HUMAN EVOLUTION RATIONALE: NOVA1 regulates alternative splic- Reintroduction of the archaic variant of NOVA1 in ing in the developing nervous system and is a master regulator of splicing genes responsible cortical organoids alters neurodevelopment for synapse formation. Altered NOVA1 splicing activity in humans is associated with neurolog- Cleber A. Trujillo, Edward S. Rice, Nathan K. Schaefer, Isaac A. Chaim, Emily C. Wheeler, Assael A. Madrigal, ical disorders, underscoring the role of NOVA1 in Justin Buchanan, Sebastian Preissl, Allen Wang, Priscilla D. Negraes, Ryan A. Szeto, Roberto H. Herai, neural function. Using CRISPR-Cas9 genome- Alik Huseynov, Mariana S. A. Ferraz, Fernando S. Borges, Alexandre H. Kihara, Ashley Byrne, editing technology in human induced pluripo- Maximillian Marin, Christopher Vollmers, Angela N. Brooks, Jonathan D. Lautz, Katerina Semendeferi, tent stem cells (iPSCs), we replaced the modern Beth Shapiro, Gene W. Yeo, Stephen E. P. Smith, Richard E. Green, Alysson R. Muotri* human allele of the NOVA1 gene with the ancestral allele found in Neanderthals and Denisovans, which contains a single-nucleotide INTRODUCTION: Current views of human evo- cies. Comparison of Neanderthal, Denisovan, substitution at position 200 that causes an lution, as supported by the fossil record, in- and extant human genomes has shown that isoleucine-to-valine change. To investigate the dicate that many hominin lineage branches many humans today carry genes introduced functional importance of this amino acid change arose, but only one survived to the present. through past admixture events and has allowed in humans, we followed iPSC neural develop- Downloaded from Neanderthals and Denisovans, two of these enumeration of human-specific genetic differ- ment through functional cortical organoids. -

New Clinical Insights and Physical Mapping of FOXG1-Regulatory Elements
European Journal of Human Genetics (2012) 20, 1216–1223 & 2012 Macmillan Publishers Limited All rights reserved 1018-4813/12 www.nature.com/ejhg ARTICLE 14q12 and severe Rett-like phenotypes: new clinical insights and physical mapping of FOXG1-regulatory elements Lila Allou1,15, Laetitia Lambert1,2,3,15, Daniel Amsallem4, Eric Bieth5, Patrick Edery6,7, Anne Destre´e8, Franc¸ois Rivier9,DavidAmor10, Elizabeth Thompson11, Julian Nicholl12, Michael Harbord13,ChristopheNemos1, Aline Saunier14, Aissa Moustaı¨ne14, Adeline Vigouroux5, Philippe Jonveaux1,14 and Christophe Philippe*,1,14 The Forkhead box G1 (FOXG1) gene has been implicated in severe Rett-like phenotypes. It encodes the Forkhead box protein G1, a winged-helix transcriptional repressor critical for forebrain development. Recently, the core FOXG1 syndrome was defined as postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and dysgenesis of the corpus callosum. We present seven additional patients with a severe Rett-like neurodevelopment disorder associated with de novo FOXG1 point mutations (two cases) or 14q12 deletions (five cases). We expand the mutational spectrum in patients with FOXG1-related encephalopathies and precise the core FOXG1 syndrome phenotype. Dysgenesis of the corpus callosum and dyskinesia are not always present in FOXG1-mutated patients. We believe that the FOXG1 gene should be considered in severely mentally retarded patients (no speech-language) with severe acquired microcephaly ( À4toÀ6 SD) and few clinical features suggestive of Rett syndrome. Interestingly enough, three 14q12 deletions that do not include the FOXG1 gene are associated with phenotypes very reminiscent to that of FOXG1-mutation-positive patients. We physically mapped a putative long-range FOXG1-regulatory element in a 0.43 Mb DNA segment encompassing the PRKD1 locus.