Fish Assemblages in the Large Lowland Narew River System (Poland): Application of the Self-Organizing Map Algorithm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deklarację Uczestnictwa W Projekcie
DEKLARACJA UCZESTNICTWA W PROJEKCIE „Aktywni razem” Numer projektu: RPPD.09.01.00-20-0232/18 Celem projektu jest realizacja PAL w Gminie Stawiski tj. wsparcia skierowanego do środowisk zagrożonych ubóstwem lub wykluczeniem społecznym, w szczególności do lokalnej społeczności na obszarach zdegradowanych, objętych rewitalizacją, odbywające się z wykorzystaniem instrumentów aktywnej integracji o charakterze: społecznym, zawodowym i zdrowotnym dla 60K i 20M zamieszkujących Gminę Stawiski w okresie do 31.07.2020r. Uczestnictwo w projekcie jest bezpłatne. Dane uczestnika projektu – dane osobowe: Imię i nazwisko …………………………………………………………….................................. PESEL: Wiek w chwili przystąpienia do projektu …………………………………….................................... Adres zamieszkania: miejscowość/ulica…………………………………………………………….. nr domu/lokalu…........................... Kod pocztowy …....…..- …..……. Gmina: Stawiski Telefon …….…………………….. e-mail……………......................………………………… W związku z uczestnictwem w projekcie: 1. Wyrażam wolę uczestnictwa w projekcie. 2. Oświadczam, iż spełniam kryteria kwalifikowalności uprawniające do udziału w projekcie. 3. Oświadczam, że zapoznałam/-em się z Regulaminem rekrutacji i uczestnictwa w projekcie „Aktywni razem” oraz akceptuję jego postanowienia. 4. W czasie trwania projektu zobowiązuję się do uczestnictwa w realizowanych zajęciach. 5. Zobowiązuję się do wypełnienia ankiet oceniających projekt, jego rezultaty działań oraz stopień realizacji celów. 6. Wyrażam zgodę na przetwarzanie danych osobowych. 7. Oświadczam, -

The Case of the Vistula River in Warsaw
Vol. 14/2010 pp. 203-212 Joanna Angiel University of Warsaw Faculty of Geography and Regional Studies Department of Geographical Education e-mail: [email protected] RESTORING THE SOCIAL VALUE OF RIVERS THROUGH EDUCATION: THE CASE OF THE VISTULA RIVER IN WARSAW Abstract: The article discusses the values of the Vistula River in Poland’s capital city, Warsaw. The author presents the results of studies identifying residents’ perceptions of the river and outlines local community processes that aim to restore the river’s social value, notably through education. Key words: public awareness, Vistula River, river value, perception, geography education. INTRODUCTION People’s relationship with rivers, and more broadly speaking with nature, has changed over time. That relationship is dependent on attitudes towards the natural environment, the cultural sphere in which an individual grows up and develops, as well as individuals’ worldviews, social awareness, and the system of received values, among other factors. (Ostrowska, 1994; Pulinowa, 1996). For many pre-agrarian societies rivers held religious, or spiritual values. They provided water, food, and protection. With the rise of agrarian societies, people remained closely tied to rivers, but the relation began to include the intensified use of waterways, chiefly for agriculture. The industrial period saw the beginnings of large-scale “industrial transfor- mations of nature,” including rivers. Deprived of their sacred status and, even, of common respect, rivers often served as channels of industrial waste disposal and recipients of urban sewage. In the present period of socio-eco- nomic development, increasingly more attention is given to the state of the environment, including rivers. -

Uchwala Nr LIII/204/18 Z Dnia 19 Kwietnia 2018 R
DZIENNIK URZĘDOWY WOJEWÓDZTWA PODLASKIEGO Białystok, dnia 27 kwietnia 2018 r. Poz. 2025 UCHWAŁA NR LIII/204/18 RADY MIEJSKIEJ W STAWISKACH z dnia 19 kwietnia 2018 r. w sprawie podziału Gminy Stawiski na stałe obwody głosowania, ustalenia ich numerów, granic oraz siedzib obwodowych komisji wyborczych Na podstawie art. 13 ust. 1 ustawy z dnia 11 stycznia 2018 r. o zmianie niektórych ustaw w celu zwiększenia udziału obywateli w procesie wybierania, funkcjonowania i kontrolowania niektórych organów publicznych (Dz. U z 2018 r., poz. 130) w związku z art. 12 § 2, 11 i 12 ustawy z dnia 5 stycznia 2011 r. Kodeks wyborczy (Dz. U z 2017 r. poz. 15 i 1089 oraz z 2018 r. poz. 4, 130 i 138) oraz art. 18 ust. 2 pkt 15 ustawy z dnia 8 marca 1990 r. o samorządzie gminnym (Dz. U z 2017 r. poz. 1875 i 2232, z 2018 r. poz. 130) uchwala się, co następuje: § 1. Dokonuje się na wniosek Burmistrza Stawisk podziału Gminy Stawiski na stałe obwody głosowania, ustala się ich numery, granice oraz siedziby obwodowych komisji wyborczych, w sposób określony w załączniku do niniejszej uchwały. § 2. Na uchwałę w sprawie podziału gminy na obwody głosowania, wyborcom w liczbie co najmniej 15 przysługuje prawo wniesienia skargi do komisarza wyborczego, w terminie 5 dni od daty podania jej do publicznej wiadomości. § 3. Wykonanie uchwały powierza się Burmistrzowi Stawisk. § 4. Po jednym egzemplarzu uchwały przekazuje się niezwłocznie Wojewodzie Podlaskiemu i Komisarzowi Wyborczemu w Łomży. § 5. Traci moc uchwała Nr XXXIII/174/13 Rady Miejskiej w Stawiskach z dnia 18 stycznia 2013 r. -

Improved Tools for River Flood Preparedness Under Changing Risk - Poland
7th Study Conference on BALTEX, Borgholm, Sweden, 10-14 June 2013 Improved tools for river flood preparedness under changing risk - Poland Zbigniew W. Kundzewicz Institute of Agricultural and Forest Environment, Polish Academy of Sciences, Poznań, Poland and Potsdam Institute for Climate Impact Research, Potsdam, Germany - Changing flood risk in Poland - Improved tools for river flood preparedness - Changing flood risk in Poland - Improved tools for river flood preparedness River flooding in Poland River flooding is the most destructive natural peril in the Baltic Sea Basin in general and in Poland in particular. Flood risk and preparedness became matters of broad concern, following the dramatic floods in Poland in 1997 and 2010, when dozens of people were killed, national flood losses reached the level of billions of Euros and the topic made it to cover stories. Floods in PL, May-June 2013 IAHS Special Publication 10 (April 2012) ISBN ISBN 978-1- 907161-28-5 (Paperback); 516 + xvi pages 10 12 14 0 2 4 6 8 Dartmouth Flood Observatory. Source: et al. (2012) Kundzewicz Dartmouth Flood Observatory. Increasing number of large floods, according tothedata in 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 y = 0.3123x + 2.46 y = 0.2608x + 0.33 R R 2 2 = 0.4339 = 0.6012 Magnitude=>5 Severity=>1.5 Trend in number of days with precipitation in excess of 30 mm, and (b) trend in maximum 5-day precipitation, 1971–2002 (after Lorenc & Olecka, 2006). Catastrophic floods of regional extent in Poland: from 1946 to 1970; from 1971 to 1990; from 1991 to 2010; from 1946 to 2010. -

Generate PDF of This Page
Institute of National Remembrance https://ipn.gov.pl/en/digital-resources/articles/7371,Under-the-red-boot.html 2021-09-29, 04:21 10.02.2021 Under the red boot It wasn’t an “ordinary” attack, because even assuming that every aggression is unique on its own, it’s hard not to notice some unprecedented aspects of the soviet invasion of Poland on September 17th, 1939. For the first time ever, one country invaded another claiming that the invaded one… didn’t exist. Narrative: abolishing of a country This was the Soviet stance on Poland on the 17th day of the German occupation, in an infamous note handed to Wacław Grzybowski, the Polish ambassador in the Soviet Union, on September 17th 1939. The note said, that Warsaw was no longer the capital of Poland and that the Polish government was disbanded. All this was supposed to mean that the Polish state “factually” ceased to exist. The Soviets went even further in creating an excuse for their aggression, claiming in a shocking document, that Poland left for itself was a place where different kinds of initiatives that could be a threat to the Soviet Union could sprout from. The “final nail in the coffin” were the note’s closing words stating that the Soviet government, unable to remain indifferent to the situation of the defenceless, “brotherly” Ukrainian and Belarusian people, ordered the Red Army to cross the Polish border and take these people under its protection. Why did Kremlin portray itself in the document as a party which wasn’t involved in the war? After all, it didn’t make a pact with Germany on the 23rd of August, 1939 to then remain neutral in the face of the German occupation of Poland. -

Floods in Poland from 1946 to 2001 — Origin, Territorial Extent and Frequency
Polish Geological Institute Special Papers, 15 (2004): 69–76 Proceedings of the Conference “Risks caused by the geodynamic phenomena in Europe” FLOODS IN POLAND FROM 1946 TO 2001 — ORIGIN, TERRITORIAL EXTENT AND FREQUENCY Andrzej DOBROWOLSKI1, Halina CZARNECKA1, Janusz OSTROWSKI1, Monika ZANIEWSKA1 Abstract. Based on the data concerning floods on the territory of Poland during the period 1946–2001, the reasons generating floods, the number of regional floods in the rivers catchment systems, and sites of local floods occurrence, were defined. Both types of floods: caused by riverbank overflows, and land flooding by rain or snow-melt water, were considered. In the most cases, the floods were caused by rainfall. They were connected with changes in the rainfall structure within Po- land. In each season of the year floods of various origin were observed. When the flood initiating factors appeared simulta- neously, the flood grew into a catastrophic size. In present analysis, for the first time in Poland, a large group of local floods has been distinguished. A special attention has been paid to floods caused by sudden flooding of the land (flash flood), including floods in the urban areas — more and more frequent during the recent years. The results of the analyses have provided important data for the assessment of the flood hazard in Poland, and for the creation of a complex flood control strategy for the whole country and/or for selected regions. Key words: flood, classification of floods, floods territorial extent, frequency of floods occurrence, torrential and rapid rain- fall, threat of life, material losses. Abstrakt. Na podstawie zbioru danych z lat 1946–2001 okreœlono przyczyny wystêpowania powodzi w Polsce, liczbê powodzi re- gionalnych w uk³adzie zlewni rzecznych oraz miejsca wyst¹pieñ powodzi lokalnych. -

Program Ochrony Środowiska Dla Gminy Juchnowiec Kościelny Do 2022 R
GMINA JUCHNOWIEC KOŚCIELNY Program Ochrony Środowiska dla Gminy Juchnowiec Kościelny do 2022 r. z perspektywą do 2026 r. 2019 r. 1 | S t r o n a Opracowanie wykonane na zlecenie: Urząd Juchnowiec Kościelny ul. Lipowa 10 16-061 Juchnowiec Kościelny www.juchnowiec.gmina.pl Wykonawca: Idenea Consulting sp. z o.o. ul. Skłodowskiej – Curie 3 lok. 63 15-094 Białystok www.idenea.pl [email protected] 2 | S t r o n a Spis treści Wykaz skrótów i symboli ......................................................................................................................... 4 1. Wstęp .............................................................................................................................................. 6 2. Streszczenie ................................................................................................................................... 10 3. Podstawowe informacje o gminie ................................................................................................. 12 3.1. Położenie i podział administracyjny .......................................................................................... 12 3.2. Budowa geologiczna, krajobraz ................................................................................................. 12 3.3. Ludność i struktura osadnicza ................................................................................................... 13 3.4. Gospodarka i rynek pracy .......................................................................................................... 14 4. -

Instytut Meteorologii I Gospodarki Wodnej
INSTYTUT METEOROLOGII I GOSPODARKI WODNEJ PAŃSTWOWY INSTYTUT BADAWCZY Biuro Prognoz Hydrologicznych w Krakowie Wydział Prognoz i Opracowań Hydrologicznych w Warszawie ul. Podleśna 61, 01-673 Warszawa tel.: 22-56-94-144 fax.: 22-834-48-97 e-mail: [email protected] www.pogodynka.pl www.imgw.pl INSTYTUT METEOROLOGII I GOSPODARKI WODNEJ PAŃSTWOWY INSTYTUT BADAWCZY BIURO PROGNOZ HYDROLOGICZNYCH W KRAKOWIE Wydział Prognoz i Opracowań Hydrologicznych w Warszawie podaje KOMUNIKAT HYDROLOGICZNY z dnia 03.10.2019 godz. 12 UTC UTC - ang. Universal Time Coordinated - czas uniwersalny; w okresie letnim czas urzedowy w Polsce UTC+2h, a w okresie zimowym UTC+1h. Stan wody Stan wody Stan wody Stan wody Stan wody Stan Stan 6 UTC 9 UTC 12 UTC 15 UTC 18 UTC Stacja ostrze- alar- Rzeka gawczy mowy obser- obser- obser- obser- obser- wodowskazowa wator czujnik wator czujnik wator czujnik wator czujnik wator czujnik [cm] A B C D E F G H I J K L M N województwo śląskie W ĄSOSZ Pilica 250 300 167 168 168 województwo świętokrzyskie Czarna JANUSZEW ICE (W łoszczowska) 320 400 274 273 274 274 województwo podlaskie SIEMIANÓW KA Narew 148 148 148 BONDARY Narew 177 177 177 NAREW Narew 170 200 46 46 46 46 PLOSKI Narew 330 370 214 213 213 SURAŻ Narew 320 340 125 125 124 124 BABINO Narew 540 570 324 324 324 STRĘKOW A GÓRA Narew 420 440 156 156 156 156 W IZNA Narew 440 470 176 176 177 177 PIĄTNICA-ŁOMŻA Narew 410 460 98 98 98 99 NOW OGRÓD Narew 360 400 48 48 49 49 BIAŁOW IEŻA - PARK Narewka 180 200 69 69 69 NAREW KA Narewka 260 290 98 98 98 CHRABOŁY Orlanka 310 350 113 -
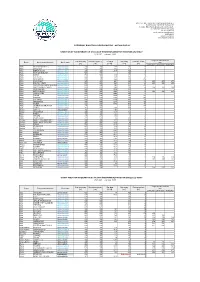
BIULETYN CODZIENNY.Pdf
INSTYTUT METEOROLOGII I GOSPODARKI WODNEJ PAŃSTWOWY INSTYTUT BADAWCZY Centralne Biuro Hydrologii Operacyjnej w Warszawie ul. Podleśna 61, 01-673 Warszawa tel.: (22) 56-94-140 e-mail: [email protected] www.imgw.pl www.meteo.imgw.pl www.stopsuszy.imgw.pl CODZIENNY BIULETYN HYDROLOGICZNY - AKTUALIZACJA* STANY WODY NA WYBRANYCH STACJACH WODOWSKAZOWYCH W DORZECZU WISŁY 27.09.2021 r. na godz. 08:00 Prognoza stanu na dzień Stan alarmowy Stan ostrzegawczy Przepływ Stan wody Dobowa zmiana Rzeka Stacja wodowskazowa Strefa stanu [cm] [cm] [cm] [m3/s] [cm] [cm] 28.09.2021 29.09.2021 30.09.2021 Wisła USTROŃ-OBŁAZIEC dolna średnich 230 180 1,83 116 -2 Wisła SKOCZÓW dolna średnich 260 210 3,26 152 -1 Wisła BIERUŃ NOWY górna średnich 330 220 25,9 109 -2 Wisła KRAKÓW-BIELANY dolna średnich 520 370 166 7 Wisła KARSY górna średnich 750 550 213 226 5 Wisła SZCZUCIN górna średnich 660 460 253 195 -2 Wisła SANDOMIERZ górna średnich 610 420 353 218 -15 Wisła ZAWICHOST dolna średnich 620 480 441 302 -16 295 280 275 Wisła PUŁAWY-AZOTY górna średnich 550 450 549 237 -14 225 220 205 Wisła WARSZAWA-NADWILANÓWKAgórna średnich 800 750 742 265 -12 Wisła WARSZAWA-BULWARY dolna średnich 650 600 740 178 -9 165 154 148 Wisła WYSZOGRÓD górna średnich 550 500 1010 376 -7 Wisła KĘPA POLSKA górna średnich 500 450 1090 311 -13 302 294 285 Wisła WŁOCŁAWEK górna średnich 650 600 1040 250 6 Wisła TORUŃ dolna średnich 650 530 1020 285 -22 Wisła FORDON dolna średnich 650 530 1060 278 -32 Wisła CHEŁMNO górna średnich 630 510 1170 331 -42 Wisła GRUDZIĄDZ górna średnich 650 540 -

Volume 18 Water Management and Protection
FACULTY OF CIVIL POLISH ASSOCIATION AND ENVIRONMENTAL ENGINEERING OF SANITARY ENGINEERS BIAŁYSTOK UNIVERSITY OF AND TECHNICIANS TECHNOLOGY SERIES OF MONOGRAPHS „ENVIRONMENTAL ENGINEERING – THROUGH A YOUNG EYE” VOLUME 18 WATER MANAGEMENT AND PROTECTION Edited by Iwona Skoczko Janina Piekutin Łukasz Malinowski Białystok 2015 FACULTY OF CIVIL POLISH ASSOCIATION AND ENVIRONMENTAL ENGINEERING OF SANITARY ENGINEERS BIAŁYSTOK UNIVERSITY OF AND TECHNICIANS TECHNOLOGY SERIES OF MONOGRAPHS VOLUME 18 WATER MANAGEMENT AND PROTECTION SERIES OF MONOGRAPHS VOLUME 18 Patronat Rektora Politechniki Białostockiej prof. dr hab. inż. Lecha Dzienisa Wydział Budownictwa i Inżynierii Środowiska Politechniki Białostockiej We would like to thank to our patronage for help in organization of this event and this publication. Sponsors: Patronage: ENVIRONMENTAL ENGINEERING – THROUGH A YOUNG EYE VOLUME 18 WATER MANAGEMENT AND PROTECTION Edited by Iwona Skoczko Janina Piekutin Łukasz Malinowski Oficyna Wydawnicza Politechniki Białostockiej Białystok 2015 Volume editor: Iwona Skoczko Janina Piekutin Łukasz Malinowski Editorial team: Iwona Skoczko – Editor-in-chef Janina Piekutin – Editor-in-chef Łukasz Malinowski – Monograph composition Reviewers of monograph: dr hab. inż. Iwona Skoczko dr n. tech. Joanna Szczykowska Scientific team: dr hab. inż. Iwona Skoczko – Politechnika Białostocka dr n. tech. Janina Piekutin – Politechnika Białostocka prof. dr hab. inż. Józefa Wiater – Politechnika Białostocka prof. dr hab. inż. Rafał Miłaszewski – Politechnika Białostocka prof. Aleksey Ternovtsev – Kijowski Narodowy Uniwersytet Budownictwa i Architektury prof. Dmitry Spitsov – Moskiewski Państwowy Uniwersytet Budownictwa prof. Javier Bayo – Uniwersytet Techniczny Cartagena dr hab. inż. Mariusz Dudziak – Politechnika Śląska dr hab. Aneta Dorota Petelska – Uniwersytet w Białymstoku prof. nzw. dr hab. Bożena Łozowicka – Instytut Ochrony Roślin dr hab. inż. Jacek Piekarski, prof. PK - Politechnika Koszalińska dr hab. -

Podziały Administracyjne Powiatów Kolneńskiego I Szczuczyńskiego (Grajewskiego) W Latach 1919-1990
Jemielity, Witold Podziały administracyjne powiatów kolneńskiego i szczuczyńskiego (grajewskiego) w latach 1919-1990 "Studia Łomżyńskie", 6, 1996, s. [141]-174 Zdigitalizowano w ramach projektu pn. Budowa platformy "Podlaskie Czasopisma Regionalne", dofinansowanego z programu „Społeczna odpowiedzialność nauki” Ministra Nauki i Szkolnictwa Wyższego (umowa SONB/SP/465121/2020). Udostępniono do wykorzystania w ramach dozwolonego użytku. Witold Jemielity (Lomza) Studia Lomżyńskie rU I11 \·1 PODZIALY ADMINISTRACYJNE POWIATÓW KOLNEŃSKIEGO I SZCZUCZYŃSKIEGO (GRAJEWSKIEGO) W LATACH 1919 - 1990. WSTĘP Opracowanie zawiera podziały administracyjne dwóch obok siebie lei..ą cych powiatów, od czasu powstania województwa białostockiego do 1990 r. Powiat kolneński istniał do 1932 r., kiedy jego oooz.ar włączono do powiatów łomżyńskiego i ostrołęckiego. W 1948 r. przywrócono powiat kolneński włą czając do niego m. in. dwie gminy powiatu grajewskiego. Powiat szczyczyń ski utrzymał swoją nazwę do 1948 r., potem był powiatem grajewskim. Punktem wyjściowym dla podziałów administracyjnych jest poszczególne miasto i konkretna wieś. Te zawsze pozostają niezmienne, gdy województwa, powiaty, gromady, gminy i sołectwa w rómych okresach obejmują większe lub mniejsze tereny, ulegają przekształceniom, nawet zanikają. W obecnym stuleciu było wiele zmian w podziale administracyjnym obu powiatów. Nikt nie opracował tego zagadnienia. Dzisiejsze podziały administracyjne są kon tynuacją dawnych, konieczna jest więc znajOOlOŚĆ tych spraw w przeszłości . Podstawę źródłową stanowią pisma urzędowe. W Dziermiku Ustaw ogła szano podziały województw, powiatów i gmin. Powtarzano to w Dziennikach Wojewódzkich dodając zmiany w gromadach i sołectwach. Od 1921 r. wy chodził Dziennik Ur~dowy Województwa Białostockiego, od 1929 r. Biało stocki Dziennik Wojewódzki, od 1950 r. Dziennik Urzędowy Wojewódzkiej Rady Narodowej w Białymstoku, od 1975 r. Dziennik Urzędowy Wojewódz• kiej Rady Narodowej w Łomży, od 1984 r. -

DRAINAGE BASIN of the BALTIC SEA Chapter 8
216 DRAINAGE BASIN OF THE BALTIC SEA Chapter 8 BALTIC SEA 217 219 TORNE RIVER BASIN 221 KEMIJOKI RIVER BASIN 222 OULUJOKI RIVER BASIN 223 JÄNISJOKI RIVER BASIN 224 KITEENJOKI-TOHMAJOKI RIVER BASINS 224 HIITOLANJOKI RIVER BASIN 226 VUOKSI RIVER BASIN 228 LAKE PYHÄJÄRVI 230 LAKE SAIMAA 232 JUUSTILANJOKI RIVER BASIN 232 LAKE NUIJAMAANJÄRVI 233 RAKKOLANJOKI RIVER BASIN 235 URPALANJOKI RIVER BASIN 235 NARVA RIVER BASIN 237 NARVA RESERVOIR 237 LAKE PEIPSI 238 GAUJA/KOIVA RIVER BASIN 239 DAUGAVA RIVER BASIN 241 LAKE DRISVYATY/ DRUKSHIAI 242 LIELUPE RIVER BASIN 245 VENTA, BARTA/BARTUVA AND SVENTOJI RIVER BASINS 248 NEMAN RIVER BASIN 251 LAKE GALADUS 251 PREGEL RIVER BASIN 254 VISTULA RIVER BASIN 260 ODER RIVER BASIN Chapter 8 218 BALTIC SEA This chapter deals with major transboundary rivers discharging into the Baltic Sea and some of their transboundary tributaries. It also includes lakes located within the basin of the Baltic Sea. TRANSBOUNDARY WATERS IN THE BASIN OF THE BALTIC SEA1 Basin/sub-basin(s) Total area (km²) Recipient Riparian countries Lakes in the basin Torne 40,157 Baltic Sea FI, NO, SE Kemijoki 51,127 Baltic Sea FI, NO, RU Oulujoki 22,841 Baltic Sea FI, RU Jänisjoki 3,861 Lake Ladoga FI, RU Kiteenjoki-Tohmajoki 1,595 Lake Ladoga FI, RU Hiitolanjoki 1,415 Lake Ladoga FI, RU Lake Pyhäjärvi and Vuoksi 68,501 Lake Ladoga FI, RU Lake Saimaa Juustilanjoki 296 Baltic Sea FI, RU Lake Nuijamaanjärvi Rakkonlanjoki 215 Baltic Sea FI, RU Urpanlanjoki 557 Baltic Sea FI, RU Saimaa Canal including 174 Baltic Sea FI, RU Soskuanjoki Tervajoki 204