Accepted Version
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Systematics of the Marine Dothideomycetes
available online at www.studiesinmycology.org StudieS in Mycology 64: 155–173. 2009. doi:10.3114/sim.2009.64.09 Molecular systematics of the marine Dothideomycetes S. Suetrong1, 2, C.L. Schoch3, J.W. Spatafora4, J. Kohlmeyer5, B. Volkmann-Kohlmeyer5, J. Sakayaroj2, S. Phongpaichit1, K. Tanaka6, K. Hirayama6 and E.B.G. Jones2* 1Department of Microbiology, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla, 90112, Thailand; 2Bioresources Technology Unit, National Center for Genetic Engineering and Biotechnology (BIOTEC), 113 Thailand Science Park, Paholyothin Road, Khlong 1, Khlong Luang, Pathum Thani, 12120, Thailand; 3National Center for Biothechnology Information, National Library of Medicine, National Institutes of Health, 45 Center Drive, MSC 6510, Bethesda, Maryland 20892-6510, U.S.A.; 4Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon, 97331, U.S.A.; 5Institute of Marine Sciences, University of North Carolina at Chapel Hill, Morehead City, North Carolina 28557, U.S.A.; 6Faculty of Agriculture & Life Sciences, Hirosaki University, Bunkyo-cho 3, Hirosaki, Aomori 036-8561, Japan *Correspondence: E.B. Gareth Jones, [email protected] Abstract: Phylogenetic analyses of four nuclear genes, namely the large and small subunits of the nuclear ribosomal RNA, transcription elongation factor 1-alpha and the second largest RNA polymerase II subunit, established that the ecological group of marine bitunicate ascomycetes has representatives in the orders Capnodiales, Hysteriales, Jahnulales, Mytilinidiales, Patellariales and Pleosporales. Most of the fungi sequenced were intertidal mangrove taxa and belong to members of 12 families in the Pleosporales: Aigialaceae, Didymellaceae, Leptosphaeriaceae, Lenthitheciaceae, Lophiostomataceae, Massarinaceae, Montagnulaceae, Morosphaeriaceae, Phaeosphaeriaceae, Pleosporaceae, Testudinaceae and Trematosphaeriaceae. Two new families are described: Aigialaceae and Morosphaeriaceae, and three new genera proposed: Halomassarina, Morosphaeria and Rimora. -

9B Taxonomy to Genus
Fungus and Lichen Genera in the NEMF Database Taxonomic hierarchy: phyllum > class (-etes) > order (-ales) > family (-ceae) > genus. Total number of genera in the database: 526 Anamorphic fungi (see p. 4), which are disseminated by propagules not formed from cells where meiosis has occurred, are presently not grouped by class, order, etc. Most propagules can be referred to as "conidia," but some are derived from unspecialized vegetative mycelium. A significant number are correlated with fungal states that produce spores derived from cells where meiosis has, or is assumed to have, occurred. These are, where known, members of the ascomycetes or basidiomycetes. However, in many cases, they are still undescribed, unrecognized or poorly known. (Explanation paraphrased from "Dictionary of the Fungi, 9th Edition.") Principal authority for this taxonomy is the Dictionary of the Fungi and its online database, www.indexfungorum.org. For lichens, see Lecanoromycetes on p. 3. Basidiomycota Aegerita Poria Macrolepiota Grandinia Poronidulus Melanophyllum Agaricomycetes Hyphoderma Postia Amanitaceae Cantharellales Meripilaceae Pycnoporellus Amanita Cantharellaceae Abortiporus Skeletocutis Bolbitiaceae Cantharellus Antrodia Trichaptum Agrocybe Craterellus Grifola Tyromyces Bolbitius Clavulinaceae Meripilus Sistotremataceae Conocybe Clavulina Physisporinus Trechispora Hebeloma Hydnaceae Meruliaceae Sparassidaceae Panaeolina Hydnum Climacodon Sparassis Clavariaceae Polyporales Gloeoporus Steccherinaceae Clavaria Albatrellaceae Hyphodermopsis Antrodiella -

Pseudodidymellaceae Fam. Nov.: Phylogenetic Affiliations Of
available online at www.studiesinmycology.org STUDIES IN MYCOLOGY 87: 187–206 (2017). Pseudodidymellaceae fam. nov.: Phylogenetic affiliations of mycopappus-like genera in Dothideomycetes A. Hashimoto1,2, M. Matsumura1,3, K. Hirayama4, R. Fujimoto1, and K. Tanaka1,3* 1Faculty of Agriculture and Life Sciences, Hirosaki University, 3 Bunkyo-cho, Hirosaki, Aomori, 036-8561, Japan; 2Research Fellow of the Japan Society for the Promotion of Science, 5-3-1 Kojimachi, Chiyoda-ku, Tokyo, 102-0083, Japan; 3The United Graduate School of Agricultural Sciences, Iwate University, 18–8 Ueda 3 chome, Morioka, 020-8550, Japan; 4Apple Experiment Station, Aomori Prefectural Agriculture and Forestry Research Centre, 24 Fukutami, Botandaira, Kuroishi, Aomori, 036-0332, Japan *Correspondence: K. Tanaka, [email protected] Abstract: The familial placement of four genera, Mycodidymella, Petrakia, Pseudodidymella, and Xenostigmina, was taxonomically revised based on morphological observations and phylogenetic analyses of nuclear rDNA SSU, LSU, tef1, and rpb2 sequences. ITS sequences were also provided as barcode markers. A total of 130 sequences were newly obtained from 28 isolates which are phylogenetically related to Melanommataceae (Pleosporales, Dothideomycetes) and its relatives. Phylo- genetic analyses and morphological observation of sexual and asexual morphs led to the conclusion that Melanommataceae should be restricted to its type genus Melanomma, which is characterised by ascomata composed of a well-developed, carbonaceous peridium, and an aposphaeria-like coelomycetous asexual morph. Although Mycodidymella, Petrakia, Pseudodidymella, and Xenostigmina are phylogenetically related to Melanommataceae, these genera are characterised by epi- phyllous, lenticular ascomata with well-developed basal stroma in their sexual morphs, and mycopappus-like propagules in their asexual morphs, which are clearly different from those of Melanomma. -

Some Additional Species of Astrosphaeriella, with a Key to the Members of the Genus
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Sydowia Jahr/Year: 1985/1986 Band/Volume: 38 Autor(en)/Author(s): Hawksworth David Leslie, Boise J. R. Artikel/Article: Some additional species of Astrosphaeriella, with a key to the members of the genus. 114-124 ©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at Sydowia, Annales Mycologici Ser. II. Vol. 38: 114-124 (1985) Verlag Ferdinand Berger & Söhne Gesellschaft m.b.H., 3580 Horn, Austria Some additional species of Astrosphaeriella, with a key to the members of the genus D. L. HAWKSWORTH Commonwealth Mycological Institute, Ferry Lane, Kew, Surrey TW9 3AF, England & J. R. BOISE New York Botanical Garden, Bronx, New York 10458, USA*) Summary. - Six species are added to the genus Astrosphaeriella SYDOW & H. SYDOW (Dothideales, Melanommataceae): A. africana D. HAWKSW. sp. nov., A. exor- rhiza BOISE sp. nov., A. minoensis (HAEA) D. HAWKSW. comb. nov. (syn. Leptosphaeria minoensis HARA), A. striaspora (E. MÜLLER) D. HAWKSW. & BOISE comb. nov. (syn. Trematosphaeria striaspora E. MÜLLER), A. tornata (BERK. & CURTIS) D. HAWKSW. & BOISE comb. nov. (syn. Sphaeria tornata BERK. & CUHTIS, IMelanornma tornatum SACC. & PAOLETTI) and A. Vesuvius (BEHK. & BROOME) D. HAWKSW. & BOISE comb. nov. (syn. Sphaeria Vesuvius BERK. & BROOME, S. agnocystis BERK. & BROOME). Tremato- sphaeria fusispora RICK is treated as a synonym of A. trochus (PENZIG & SACC.) D. HAWKSW. Some additional collections seen are mentioned and a key is provided to the ten species accepted as members of the genus. Introduction The genus Astrosphaeriella SYDOW & H. SYDOW (Dothideales, Melanommataceae) was re-introduced by HAWKSWORTH (1981) for four species, characteristically occurring on the petioles of palms and bamboo culms in the tropics. -

Paraconiothyrium, a New Genus to Accommodate the Mycoparasite Coniothyrium Minitans, Anamorphs of Paraphaeosphaeria, and Four New Species
STUDIES IN MYCOLOGY 50: 323–335. 2004. Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species 1* 2 3 1 Gerard J.M. Verkley , Manuela da Silva , Donald T. Wicklow and Pedro W. Crous 1Centraalbureau voor Schimmelcultures, Fungal Biodiversity Centre, PO Box 85167, NL-3508 AD Utrecht, the Netherlands; 2Fungi Section, Department of Microbiology, INCQS/FIOCRUZ, Av. Brasil, 4365; CEP: 21045-9000, Manguinhos, Rio de Janeiro, RJ, Brazil. 3Mycotoxin Research Unit, National Center for Agricultural Utilization Research, 1815 N. University Street, Peoria, IL 61604, Illinois, U.S.A. *Correspondence: Gerard J.M. Verkley, [email protected] Abstract: Coniothyrium-like coelomycetes are drawing attention as biological control agents, potential bioremediators, and producers of antibiotics. Four genera are currently used to classify such anamorphs, namely, Coniothyrium, Microsphaeropsis, Cyclothyrium, and Cytoplea. The morphological plasticity of these fungi, however, makes it difficult to ascertain their best generic disposition in many cases. A new genus, Paraconiothyrium is here proposed to accommodate four new species, P. estuarinum, P. brasiliense, P. cyclothyrioides, and P. fungicola. Their formal descriptions are based on anamorphic characters as seen in vitro. The teleomorphs of these species are unknown, but maximum parsimony analysis of ITS and partial SSU nrDNA sequences showed that they belong in the Pleosporales and group in a clade including Paraphaeosphaeria s. str., the biocontrol agent Coniothyrium minitans, and the ubiquitous soil fungus Coniothyrium sporulosum. Coniothyrium minitans and C. sporulosum are therefore also combined into the genus Paraconiothyrium. The anamorphs of Paraphaeosphaeria michotii and Paraphaeosphaeria pilleata are regarded representative of Paraconiothyrium, but remain formally unnamed. -
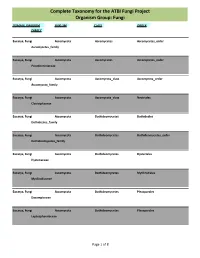
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Ascomycetes_family Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Pseudeurotiaceae Eucarya, Fungi Ascomycota Ascomycota_class Ascomycota_order Ascomycota_family Eucarya, Fungi Ascomycota Ascomycota_class Nectriales Clavicipitaceae Eucarya, Fungi Ascomycota Dothideomycetes Dothideales Dothideales_family Eucarya, Fungi Ascomycota Dothideomycetes Dothideomycetes_order Dothideomycetes_family Eucarya, Fungi Ascomycota Dothideomycetes Hysteriales Hysteriaceae Eucarya, Fungi Ascomycota Dothideomycetes Mytilinidiales Mytilinidiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Dacampiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Leptosphaeriaceae Page 1 of 8 Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Lophiostomataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Massarinaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Melanommataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleomassariaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales_family Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Sporormiaceae Eucarya, Fungi Ascomycota Dothideomycetes -

Pseudotrichia Viburnicola (P
Pseudotrichia viburnicola (P. Crouan & H. Crouan) Rossman Fungi, Ascomycota, Pezizomycotina, Dothideomycetes, Pleosporomycetidae, Pleosporales, Melanommataceae 19.08.2010 Heidelberg/Germany, southern bank of the Neckar river, „Chestnut Terrace“ east of the „Alte Brücke“, (49° 24‘ 49.61“ N, 8° 42‘ 42.46“ E). On a small dead twig of Aesculus hippocastanum, partly decorticated, on the ground. Ascocarps nearly unvisible without loupe in dry state. Rehydrated ascocarps. Topview of an ascocarp. Sideview and section of a mature ascocarp. Pseudothecia c. 1mm/dm, immersed in the bark or decorticated wood. The orange neck is protuding and of- ten ornamented with white hairs. Mature ascocarps feature a lump of hyphen atop the ostiolum covered with ejected spores. The peridium is soft, gelatinous, width of the wall c. 120 µm. Asci. Spores with 7 septa, 54-70 x 10,1-12,5 µm Bibliography: Crouan, P.L./Crouan, H.M., Florule de Finistère, contenant des descriptions de 360 espèces nouvelles de sporogames, des nombreuses observations, Paris/Brest 1867, 39. Rossman, A.Y., A preliminary account of the taxa described in Calonectria, Mycotaxon 8, 1979, 550. Rossman, A.Y.,The Tubeufiaceae and similar Loculoascomycetes, Mycological Papers 157, 1987, 6. Leroy, P./Pacaud, R., Trois Ascomycota rares ou intéressants de Vendée (France): Contribution n°37 au Programme national d‘inventaire mycologique et de cartographie des Mycota français, Documents Mycolo- giques, nouvelle série, XXX(117-118) 2000, 115-117, Pl. Ia. Other finds (in chronological order): 1 - 19th cent., Finistère, France, Viburnum tinus, (Holotype, Crouan&Crouan) 2 - 27.06.1987, Sorges (Angers), Dept. 49, France, Lonicera, leg. R. -

Splanchnonema-Like Species in Pleosporales: Introducing Pseudosplanchnonema Gen
Phytotaxa 231 (2): 133–144 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2015 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.231.2.2 Splanchnonema-like species in Pleosporales: introducing Pseudosplanchnonema gen. nov. in Massarinaceae K.W. THILINI CHETHANA1,2,3, MEI LIU1, HIRAN A. ARIYAWANSA2,3, SIRINAPA KONTA2,3, DHANUSHKA N. WANASINGHE2,3, YING ZHOU1, JIYE YAN1, ERIO CAMPORESI4, TIMUR S. BULGAKOV5, EKACHAI CHUKEATIROTE2,3, KEVIN D. HYDE2,3, ALI H. BAHKALI6, JIANHUA LIU1,* & XINGHONG LI1,* 1Institute of Plant and Environment Protection, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, China 2Institute of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai, 57100, Thailand 3School of Science, Mae Fah Luang University, Chiang Rai, 57100, Thailand 4A.M.B. Gruppo Micologico Forlivese “A.M.B. Gruppo Micologico Forlivese “Antonio Cicognani”, Via Roma 18, Forlì, Italy; A.M.B. Circolo Micologico “Giovanni Carini”, C.P. 314, Brescia, Italy; Società per gli Studi Naturalistici della Romagna, C.P. 144, Bagnaca- vallo (RA), Italy 5Academy of Biology and Biotechnology, Southern Federal University, Rostov-on-Don 344090, Russia 6 Botany and Microbiology Department, College of Science, King Saud University, Riyadh, KSA 11442, Saudi Arabia. * e-mail: [email protected] (J.H. Liu), [email protected] (X. H. Li) Abstract In this paper we introduce a new genus Pseudosplanchnonema with P. phorcioides comb. nov., isolated from dead branches of Acer campestre and Morus species. The new genus is confirmed based on morphology and phylogenetic analyses of se- quence data. Phylogenetic analyses based on combined LSU and SSU sequence data showed that P. -

Proposed Generic Names for Dothideomycetes
Naming and outline of Dothideomycetes–2014 Nalin N. Wijayawardene1, 2, Pedro W. Crous3, Paul M. Kirk4, David L. Hawksworth4, 5, 6, Dongqin Dai1, 2, Eric Boehm7, Saranyaphat Boonmee1, 2, Uwe Braun8, Putarak Chomnunti1, 2, , Melvina J. D'souza1, 2, Paul Diederich9, Asha Dissanayake1, 2, 10, Mingkhuan Doilom1, 2, Francesco Doveri11, Singang Hongsanan1, 2, E.B. Gareth Jones12, 13, Johannes Z. Groenewald3, Ruvishika Jayawardena1, 2, 10, James D. Lawrey14, Yan Mei Li15, 16, Yong Xiang Liu17, Robert Lücking18, Hugo Madrid3, Dimuthu S. Manamgoda1, 2, Jutamart Monkai1, 2, Lucia Muggia19, 20, Matthew P. Nelsen18, 21, Ka-Lai Pang22, Rungtiwa Phookamsak1, 2, Indunil Senanayake1, 2, Carol A. Shearer23, Satinee Suetrong24, Kazuaki Tanaka25, Kasun M. Thambugala1, 2, 17, Saowanee Wikee1, 2, Hai-Xia Wu15, 16, Ying Zhang26, Begoña Aguirre-Hudson5, Siti A. Alias27, André Aptroot28, Ali H. Bahkali29, Jose L. Bezerra30, Jayarama D. Bhat1, 2, 31, Ekachai Chukeatirote1, 2, Cécile Gueidan5, Kazuyuki Hirayama25, G. Sybren De Hoog3, Ji Chuan Kang32, Kerry Knudsen33, Wen Jing Li1, 2, Xinghong Li10, ZouYi Liu17, Ausana Mapook1, 2, Eric H.C. McKenzie34, Andrew N. Miller35, Peter E. Mortimer36, 37, Dhanushka Nadeeshan1, 2, Alan J.L. Phillips38, Huzefa A. Raja39, Christian Scheuer19, Felix Schumm40, Joanne E. Taylor41, Qing Tian1, 2, Saowaluck Tibpromma1, 2, Yong Wang42, Jianchu Xu3, 4, Jiye Yan10, Supalak Yacharoen1, 2, Min Zhang15, 16, Joyce Woudenberg3 and K. D. Hyde1, 2, 37, 38 1Institute of Excellence in Fungal Research and 2School of Science, Mae Fah Luang University, -

Pleosporales, Dothideomycetes)
Mycosphere 5 (3): 411–417 (2014) ISSN 2077 7019 www.mycosphere.org Article Mycosphere Copyright © 2014 Online Edition Doi 10.5943/mycosphere/5/3/3 A new species, Lophiostoma versicolor, from Japan (Pleosporales, Dothideomycetes) Hirayama K1, Hashimoto A2, 3 and Tanaka K2 1 Apple Experiment Station, Aomori Prefectural Agriculture and Forestry Research Center, 24 Fukutami, Botandaira, Kuroishi, Aomori 036-0332, Japan 2 Faculty of Agriculture and Life Sciences, Hirosaki University, 3 Bunkyo-cho, Hirosaki, Aomori, 036-8561, Japan 3The United Graduate School of Agricultural Sciences, Iwate University, 18-8 Ueda 3 chome, Morioka 020-8550, Japan Hirayama K, Hashimoto A, Tanaka K 2014 – A new species, Lophiostoma versicolor, from Japan (Pleosporales, Dothideomycetes). Mycosphere 5(3), 411–417, Doi 10.5943/mycosphere/5/3/3 Abstract Lophiostoma versicolor sp. nov. was found on Acer sp. in Japan. This species is characterized by ascomata with a laterally compressed apex; clavate, 2(–4)-spored asci with a long stipe; and verruculose, 3-septate, versicolored ascospores without a sheath or appendages. Phylogenetic analyses based on LSU nrDNA sequences supported the generic placement and species validity of L. versicolor. Key words – ITS – Lophiostomataceae – Lophiotrema – LSU nrDNA – Pleosporomycetidae – Systematics – Taxonomy Introduction During an investigation of bitunicate ascomycetes in Japan, an unidentified fungus was found on dead twigs of Acer sp. The morphological characteristics of the fungus, such as the presence of ascomata with a compressed beak and clavate asci, recall those of Lophiostoma (Hirayama & Tanaka 2011) belonging to the Lophiostomataceae. This fungus, however, is different from any of the existing species of the genus because it possesses 2(–4)-spored asci and verruculose, 3-septate, versicolored ascospores without a sheath or appendages. -

Fungi from Palms. XXXIX. Asymmetricospora Gen
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Sydowia Jahr/Year: 1998 Band/Volume: 50 Autor(en)/Author(s): Fröhlich Jane, Hyde Kevin D. Artikel/Article: Fungi from palms. XXXIX. Asymmetricospora gen. et sp. nov. (Melanommataceae). 182-186 ©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at Fungi from palms. XXXIX. Asymmetricospora gen. et sp. nov. (Melanommataceae) Jane Fröhlich1 & Kevin D. Hyde2 1 Manaaki Whenua, Landcare Research New Zealand Ltd, Private Bag 92170, Auckland, New Zealand 2 Fungal Diversity Research Project, Department of Ecology and Biodiversity, The University of Hong Kong, Pokfulam Road, Hong Kong Fröhlich, J. & K. D. Hyde (1998). Fungi from palms. XXXIX. Asymme- tricospora gen. et sp. nov. (Melanommataceae). - Sydowia 50(2): 182 186. The genus Asymmetricospora (Melanommataceae, Melannomatales) is in- troduced here to accommodate a single species, A. calamicola, which has immersed ascomata, fissitunicate asci and a hamathecium of trabeculae. It is separated from other Melanommataceous genera by the absence of a subiculum, the absence of short dark setae around the papilla, and its asymmetric ascospore morphology. Astrosphaeriella is the most similar in overall appearance, but Asymmetricospora differs in ostiole and ascospore morphology and in the coalescing ascomata (i.e. the fruiting bodies of Asymmetricospora calamicola contain more than one locule, those of Astrosphaeriella normally one). Keywords: Astrosphaeriella, new species, Melanommatales. We are investigating the fungi occurring on palms and in this paper we describe a new genus, Asymmetricospora, to accommodate a single species, A. calamicola. The species keys out to the Mela- nommataceae (Melanommatales sensu Barr, 1990) on the basis of its immersed ascomata, ostiole and peridium morphology, fissitunicate asci and trabeculate pseudoparaphyses (Barr, 1990). -

Mycosphere Notes 169–224 Article
Mycosphere 9(2): 271–430 (2018) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/9/2/8 Copyright © Guizhou Academy of Agricultural Sciences Mycosphere notes 169–224 Hyde KD1,2, Chaiwan N2, Norphanphoun C2,6, Boonmee S2, Camporesi E3,4, Chethana KWT2,13, Dayarathne MC1,2, de Silva NI1,2,8, Dissanayake AJ2, Ekanayaka AH2, Hongsanan S2, Huang SK1,2,6, Jayasiri SC1,2, Jayawardena RS2, Jiang HB1,2, Karunarathna A1,2,12, Lin CG2, Liu JK7,16, Liu NG2,15,16, Lu YZ2,6, Luo ZL2,11, Maharachchimbura SSN14, Manawasinghe IS2,13, Pem D2, Perera RH2,16, Phukhamsakda C2, Samarakoon MC2,8, Senwanna C2,12, Shang QJ2, Tennakoon DS1,2,17, Thambugala KM2, Tibpromma, S2, Wanasinghe DN1,2, Xiao YP2,6, Yang J2,16, Zeng XY2,6, Zhang JF2,15, Zhang SN2,12,16, Bulgakov TS18, Bhat DJ20, Cheewangkoon R12, Goh TK17, Jones EBG21, Kang JC6, Jeewon R19, Liu ZY16, Lumyong S8,9, Kuo CH17, McKenzie EHC10, Wen TC6, Yan JY13, Zhao Q2 1 Key Laboratory for Plant Biodiversity and Biogeography of East Asia (KLPB), Kunming Institute of Botany, Chinese Academy of Science, Kunming 650201, Yunnan, P.R. China 2 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 3 A.M.B. Gruppo Micologico Forlivese ‘‘Antonio Cicognani’’, Via Roma 18, Forlı`, Italy 4 A.M.B. Circolo Micologico ‘‘Giovanni Carini’’, C.P. 314, Brescia, Italy 5 Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Science, Kunming 650201, Yunnan, P.R. China 6 Engineering and Research Center for Southwest Bio-Pharmaceutical Resources of national education Ministry of Education, Guizhou University, Guiyang, Guizhou Province 550025, P.R.