Epidural Anesthesia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study Protocol and Statistical Analysis Plan
The University of Texas Southwestern Medical Center at Dallas Institutional Review Board PROJECT SUMMARY Study Title: Ultrasound-guided fascia iliaca compartment block versus periarticular infiltration for pain management after total hip arthroplasty: a randomized controlled trial Principal Investigator: Irina Gasanova, MD Sponsor/Funding Source: Department of Anesthesiology and Pain Management, UT Southwestern Medical School IRB Number: STU 122015-022 NCT Number: NCT02658240 Date of Document: 01 April 2016 Page 1 of 7 Purpose: In this randomized, controlled, observer-blinded study we plan to evaluate ultrasound-guided fascia iliaca compartment block with ropivacaine and periarticular infiltration with ropivacaine for postoperative pain management after total hip arthroplasty (THA). Background: Despite substantial advances in our understanding of the pathophysiology of pain and availability of newer analgesic techniques, postoperative pain is not always effectively treated (1). Optimal pain management technique balances pain relief with concerns about safety and adverse effects associated with analgesic techniques. Currently, postoperative pain is commonly treated with systemic opioids, which are associated with numerous adverse effects including nausea and vomiting, dizziness, drowsiness, pruritus, urinary retention, and respiratory depression (2). Use of regional and local anesthesia has been shown to reduce opioid requirements and opioid-related side effects. Therefore, their use has been emphasized (3, 4, 5, 6). Fascia Iliaca compartment block (FICB) is a field block that blocks the nerves from the lumbar plexus supplying the thigh (i.e., lateral femoral cutaneous femoral and obturator nerves). The obturator nerve is sometimes involved in the FICB but probably plays little role in postoperative pain relief for most surgeries of the hip and proximal femur. -

Product Catalog POLICIES Ordering Options Payment Methods
Product Catalog POLICIES Ordering Options Payment Methods Three Easy Ways To Order Payment Portal and ACH Visit www.sunsethcs.com/paymentportal to pay online with Phone ACH or credit card. (877) 5-SUNSET (877-578-6738) Our customer service professionals are here to assist you Net 30-Day Payment Terms M-F 8:00 AM – 5:00 PM Central Time. For qualified companies. A 1.5% monthly finance charge will be assessed on all past due invoices. Fax 312-997-9985 — Accepted 24 hours a day. COD For non-qualified companies. Email [email protected] Credit Card We accept all major credit cards. A processing fee may apply. After 10 days of invoice date, a surcharge of 2.5% will be applied. Shipping Customer Service Drop shipping available — ask your sales rep Returns about sending your order to multiple locations. Call us for a return authorization number. There is no restocking charge on orders returned within 20 days of UPS invoice. Orders returned after 20 days may be assessed a Whenever possible, orders ship via UPS. If another carrier restocking fee of 15%. No returns after 90 days. is desired, please contact your sales rep. (877) 5-SUNSET (877-578-6738) All orders are packed in the smallest boxes available and Central Distribution Center Western Distribution Center combined with other products on the order to reduce Sunset Healthcare Solutions Sunset Healthcare Solutions shipping costs. 279 Madsen Dr Ste 101 2450 Statham Blvd Bloomingdale, IL 60108 Oxnard, CA 93033 All products ship prepaid and are added to the invoice. Problems All products ship from Bloomingdale, IL or Oxnard, CA. -

Frequently Asked Questions (Faqs)
Frequently Asked Questions (FAQs) What is hyperbaric oxygen therapy? Commonly referred to as HBOT, hyperbaric oxygen therapy enhances the body’s natural healing process by delivering oxygen under pressure, which increases the oxygen content in the blood, plasma, cerebral spinal fluid, and other body tissues. There are two basic types of HBOT—hard HBOT and mild HBOT. With hard HBOT, treatments are delivered in a hard-sided chamber typically at pressures greater than 1.5 ATA and using 100% oxygen. 100% oxygen is extremely flammable; therefore, hard HBOT involves managing the risk of explosion. Another concern with hard HBOT is oxygen toxicity. While hard HBOT with 100% oxygen results in greater oxygen saturation in the tissues, many conditions respond better to mild HBOT. In clinical trials to date, there has been virtually no difference in clinical outcome between mild HBOT and hard HBOT. Mild HBOT refers to hyperbaric oxygen therapy at lower pressures, typically 1.5 ATA or below, and the use of an oxygen concentrator delivering 90-95% oxygen inside a portable soft-sided chamber. Mild HBOT has no known safety risks with fire or toxicity, and it is substantially less expensive. Our facility provides concentrated oxygen (90-95%) at 1.3 ATA—(Mild HBOT)a highly effective combination clinically, and without the risk of oxygen toxicity or explosion, as 100% oxygen is avoided. How should I expect mild HBOT to feel? You will be seated or lying down inside the chamber, relaxing comfortably in your own clothing, as you breathe concentrated oxygen (90-95% O2) through a facemask. -

Stratus 5 Oxygen Concentrator User Manual
USER MANUAL OXYGEN CONCENTRATOR CE 0123 v2.1 STR1005 User Manual Symbol Key MARK DEFINITION II Power on Power off Follow Instruction for Use No smoking Caution, consult accompanying documents. Class Ⅱ (Double Insulated) Type BF Applied Part CE certification mark 0123 AC Power Stacking Limit by Number This Way Up Fragile, handle with care Keep dry Temperature limit No open flames IIPP2211 IP21 Drip Proof Equipment Consult instructions for use Stand-by Warning, electricity 2 v2.1 STR1005 User Manual 3 v2.1 STR1005 User Manual SPECIAL NOTES • Please read this manual carefully before using this product and save it for future reference. • If you need assistance with this manual, Please contact your local DME or home health provider • The Stratus 5 is a prescription device. Use only the liter setting prescribed for you. • It is always recommended for critically ill patients to have a backup oxygen source in case of malfunction. • If patient experiences an adverse reaction contact physician or call 911 immediately. • In case of machine malfunction, contact the home medical equipment provider; do not attempt to disassemble the Stratus 5. • The Stratus 5 is not intended as life support, it is for supplemental oxygen use only. Patients with special needs may be unable to understand the alarm features and should be well supervised while using an oxygen concentrator. • The Stratus 5 is for single patient use. • Do not adjust the flowmeter float beyond the red line position. Long-term use out of range will reduce the efficiency of the oxygen generator. SAFETY NOTICE Please read the following information carefully before Operating the oxygen concentrator Warning Special attention should be paid to reducing the risk of fire when using oxygen therapy. -

Epidural Analgesia Guidelines for the Rhw
LOCAL OPERATING PROCEDURE CLINICAL POLICIES, PROCEDURES & GUIDELINES Approved by Quality & Patient Care Committee 2 June 2016 EPIDURAL ANALGESIA GUIDELINES FOR THE RHW SECTION 1 RATIONAL SECTION 2 EDUCATION OF NURSING/MIDWIFERY STAFF SECTION 3 INDICATIONS/DOSING/OBSERVATIONS SECTION 4 PROCEDURE SECTION 5 MANAGEMENT GUIDELINES SECTION 6 COMPLICATIONS & MANAGEMENT SECTION 7 REMOVAL OF EPIDURAL CATHETER SECTION 8 CONCURRENT USE OF ANTICOAGULANTS APPENDIX 1 EPIDURAL DISCHARGE ADVICE APPENDIX 2 PATIENT DISCHARGE INSTRUCTIONS AFTER EPIDURAL BLOOD PATCH …./2 2. LOCAL OPERATING PROCEDURE CLINICAL POLICIES, PROCEDURES & GUIDELINES Approved by Quality & Patient Care Committee 2 June 2016 EPIDURAL ANALGESIA GUIDELINES FOR THE RHW cont’d SECTION 1 – RATIONAL • The blockade of transmission of pain impulses by the use of local anaesthetic medication can reduce the body’s physiological response to the stress of pain. • Systemic opioids only, although a strong pain reliever, may cause respiratory depression, sedation, nausea, vomiting, confusion, lightheadedness, constipation and immobilisation. • The goal of regional axial blockade (epidural analgesia) in moderate to severe pain is to diminish the development of an efficient pain pathway, by blocking conduction along pain nerve fibers. • Epidural infusions however, requires constant assessment and at times intervention in order to provide this level of pain control. • Vigilance is required as tolerance to local anaesthetic can develop which can require more agent be infused in order to maintain the level of block. • Other factors such as patient position and movement will influence the effectiveness of the infusion, as will the precision of the pump and time spent when changing infusions. • Opioids added to an epidural infusion can augment the analgesic effect of the local anaesthetic block. -
Oxymask™ Empower Best Practice
OxyMask™ Empower Best Practice. Safer Care. Exceptional Experience. Clinical Evidence Summary Executive Summary OxyMask is a solution for safer, more efficient oxygen delivery. It is an all-in-one replacement for other oxygen delivery modalities, such as the venturi mask and non-rebreather mask. The unique OxyMask technology helps enhance patient experience, increase efficiency, and reduce costs. Introduction: Enhance Patient Experience In an ever-competitive healthcare market, patient experience is at the forefront of advances in healthcare. Patients treated with oxygen therapy may feel they are trapped or helpless.1 Patient-centered solutions are increasingly recommended in policy and research.2 OxyMask brings patient-centered care to fruition through its design and dependability. Patient-centered design OxyMask utilizes a light-weight, open design that: • Sits lightly on the face • Allows unrestricted communication • Allows for oral medication delivery • May reduce the feeling of claustrophobia Dependability OxyMask is clinically proven to deliver more oxygen at lower flow rates compared to traditional delivery methods.3 Additionally, laboratory testing suggests that OxyMask reduces the risk of carbon dioxide rebreathing when compared to non-rebreather masks.4,5 OxyMask™ OxyMask Delivers Oxygen More Efficiently OxyMask May Reduce the Risk of Carbon Than a Venturi Mask3 Dioxide Rebreathing Compared to a Beecroft JM, Hanly PJ. Comparison of the OxyMask and Venturi mask in the Non-Rebreather Mask4 delivery of supplemental oxygen: Pilot study in oxygen-dependent patients. Can Respir J. 2006;13(5):247-252. Lamb K, Piper D. Southmedic OxyMaskTM compared with the Hudson RCI® Non-Rebreather Mask™: Safety and performance comparison. Can J Resp Ther. -
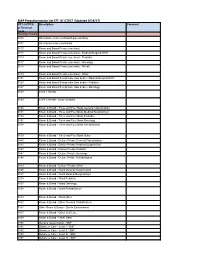
MAP Preauthorization List EFF: 8/1/2017 (Updated 8/24/17)
MAP Preauthorization List EFF: 8/1/2017 (Updated 8/24/17) CPT, HCPCS Description Comment or Revenue Code Revenue Codes 0100 All inclusive room and board plus ancillary 0101 All inclusive room and board 0110 Room and Board Private (one bed) 0111 Room and Board Private (one bed) - Medical/Surgical/GYN 0113 Room and Board Private (one bed) - Pediatric 0117 Room and Board Private (one bed) - Oncology 0118 Room and Board Private (one bed) - Rehab 0119 Room and Board Private (one bed) - Other 0121 Room and Board Semiprivate (two beds) - Medical/Surgical/GYN 0123 Room and Board Semiprivate (two beds) - Pediatric 0127 Room and Board Semiprivate (two beds) - Oncology 0128 Level 1 Rehab 0129 Level 2 Rehab - acute complex 0130 Room & Board - Three and Four Beds General Classification 0131 Room & Board - Three and Four Beds Medical/Surgical/Gyn 0133 Room & Board - Three and Four Beds Pediatric 0137 Room & Board - Three and Four Beds Oncology 0138 Room & Board - Three and Four Beds Rehabilitation 0139 Room & Board - Three and Four Beds Other 0140 Room & Board - Deluxe Private General Classification 0141 Room & Board - Deluxe Private Medical/Surgical/Gyn 0143 Room & Board - Deluxe Private Pediatric 0147 Room & Board - Deluxe Private Oncology 0148 Room & Board - Deluxe Private Rehabilitation 0149 Room & Board - Deluxe Private Other 0150 Room & Board - Ward General Classification 0151 Room & Board - Ward Medical/Surgical/Gyn 0153 Room & Board - Ward Pediatric 0157 Room & Board - Ward Oncology 0158 Room & Board - Ward Rehabilitation 0159 Room & Board - -

Ultrasound-Guided Fascia Iliaca Compartment Block (FICB)
Ultrasound-Guided Fascia Iliaca Compartment Block (FICB) Hip Fracture Any of the following present? 1) Neurologic deficit 2) Multisystem Trauma 3) Allergy to local anesthetic Yes *Anticoagulated patients – physician No discretion Standard Care Procedure Details 1) Document distal neurovascular exam in EPIC 1) PO acetaminophen 2) Consult ortho (do not need to await callback) (1,000mg) 3) Obtain verbal consent from patient 2) Consider 0.1 mg/kg 4) Position Patient and U/S Machine morphine or opioid 5) Order Bupivacaine (dose dependent) a. Max 2 mg/kg equivalent b. i.e. 100 mg safe for 50 kg patient 3) Screening labs, CXR, 6) Standard ASA monitoring (telemetry during procedure with ECG, type and screen continuous pulse oximetry, BP measurement, and IV access) 7) Perform FICB 4) Consult orthopedics and medicine as 8) Inform patients on block characteristics: a. Onset ~ 20 minutes necessary b. Duration ~ 8-12 hours Equipment Required Counseling Ultrasound Machine 1) Linear/Vascular Probe set to “Nerve” Benefits setting for best results Decreases: Anesthetic: 1) Pain 1) 0.5% Bupivacaine (20-30 cc) 2) 1% Lidocaine (3-5 cc) 2) Delirium 3) Opioids Saline (10 cc) 4) Hypoxia Syringes: 1) 30 cc 2) 3-5 cc Risks Needles: 1) Pain at injection site 1) Blunt fill 2) Temporary nerve palsy 2) 25G 3) 18G LP needle 3) Intravascular injection Chloroprep or Alcohol swab(s) 4) Local Anesthetic Systemic Toxicity * (LAST) * LAST (local anesthetic systemic toxicity) 1) Rare and only with intravascular injection which an ultrasound guided approach prevents. 2) Signs include arrhythmias, seizures, convulsions 3) Treatment a. -

Herestraat 49, B-3000 Leuven Yves Kremer, CU Saint-Luc, Av
Editor | Prof. Dr. V. Bonhomme CO-Editors | Dr. Y. Kremer — Prof. Dr. M. Van de Velde ACTA ANAESTHESIOLOGICA JOURNAL OF THE BELGIAN SOCIETY OF ANESTHESIOLOGY, RESUSCITATION, PERIOPERATIVE MEDICINE AND PAIN MANAGEMENT (BeSARPP) BELGICA Indexed in EMBASE l EXCERPTA MEDICA ISSN: 2736-5239 Suppl. 1 202071 Master Theses www.besarpp.be Cover-1 -71/suppl.indd 1 12/01/2021 12:37 ACTA ANÆSTHESIOLOGICA BELGICA 2020 – 71 – Supplement 1 EDITORS Editor-in-chief : Vincent Bonhomme, CHU Liège, av. de l’Hôpital 1, B-4000 Liège Co-Editors : Marc Van de Velde, KU Leuven, Herestraat 49, B-3000 Leuven Yves Kremer, CU Saint-Luc, av. Hippocrate, B-1200 Woluwe-Saint-Lambert Associate Editors : Margaretha Breebaart, UZA, Wilrijkstraat 10, B-2650 Edegem Christian Verborgh, UZ Brussel, Laarbeeklaan 101, B-1090 Jette Fernande Lois, CHU Liège, av. de l’Hôpital 1, B-4000 Liège Annelies Moerman, UZ Gent, C. Heymanslaan 10, B-9000 Gent Mona Monemi, CU Saint-Luc, av. Hippocrate, B-1200 Woluwe-Saint-Lambert Steffen Rex, KU Leuven, Herestraat 49, B-3000 Leuven Editorial assistant Carine Vauchel Dpt of Anesthesia & ICM, CHU Liège, B-4000 Liège Phone: 32-4 321 6470; Email: [email protected] Administration secretaries MediCongress Charlotte Schaek and Astrid Dedrie Noorwegenstraat 49, B-9940 Evergem Phone : +32 9 218 85 85 ; Email : [email protected] Subscription The annual subscription includes 4 issues and supplements (if any). 4 issues 1 issue (+supplements) Belgium 40€ 110€ Other Countries 50€ 150€ BeSARPP account number : BE97 0018 1614 5649 - Swift GEBABEBB Publicity : Luc Foubert, treasurer, OLV Ziekenhuis Aalst, Moorselbaan 164, B-9300 Aalst, phone: +32 53 72 44 61 ; Email : [email protected] Responsible Editor : Prof. -

E-Cigarette Use in Patients Receiving Home Oxygen Therapy
FOCUSED REVIEW E-cigarette use in patients receiving home oxygen therapy Yves Lacasse MD MSc FRCP1,2, Martin Légaré MD FRCP3, François Maltais MD FRCP1,2 Y Lacasse, M Légaré, F Maltais. E-cigarette use in patients receiving La cigarette électronique chez les patients sous home oxygen therapy. Can Respir J 2015;22(2):83-85. oxygénothérapie à domicile Current smokers who are prescribed home oxygen may not benefit from the therapy. In addition to being an obvious fire hazard, there is some evi- Il se peut que les fumeurs qui se font prescrire une oxygénothérapie à domi- dence that the physiological mechanisms by which home oxygen is cile ne profitent pas de ce traitement. Sans compter que le tabagisme pose believed to operate are inhibited by smoking. Although their effectiveness un risque d’incendie évident, certaines données probantes indiquent qu’il is yet to be demonstrated, electronic cigarettes (e-cigarettes) are often inhibe les mécanismes physiologiques par lesquels l’oxygénothérapie à regarded as an aid to smoking cessation. However, several burn accidents domicile fonctionnerait. Même si son efficacité reste à démontrer, la ciga- in e-cigarette smokers receiving home oxygen therapy have also been rette électronique (vapoteuse) est souvent perçue comme une aide au reported, leading Health Canada to release a warning of fire risk to oxygen sevrage du tabagisme. Cependant, plusieurs incidents de brûlure chez des therapy patients from e-cigarettes. It is the authors’ position that patients vapoteurs sous oxygénothérapie à domicile ont été déclarés, ce qui a incité receiving oxygen should definitely not use e-cigarettes. -

Summary of Product Characteristics
Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT /…/ 2 mg/ml solution for injection /…/ 7.5 mg/ml solution for injection /…/ 10 mg/ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION /…/ 2 mg/ml solution for injection: 1 ml contains 2.12 mg ropivacaine hydrochloride monohydrate, equivalent to 2 mg of ropivacaine hydrochloride. Excipient with know effect: sodium 3.6 mg/ml 10 ml amp. contains 21.2 mg ropivacaine hydrochloride monohydrate, equivalent to 20 mg of ropivacaine hydrochloride. 20 ml amp. contains 42.3 mg ropivacaine hydrochloride monohydrate, equivalent to 40 mg of ropivacaine hydrochloride. /…/ 7.5 mg/ml solution for injection: 1 ml contains 7.94 mg ropivacaine hydrochloride monohydrate, equivalent to 7.5 mg of ropivacaine hydrochloride. Excipient with known effect: sodium 3.0 mg/ml 10 ml amp. contains 79.4 mg ropivacaine hydrochloride monohydrate, equivalent to 75 mg of ropivacaine hydrochloride. 20 ml amp. contains 158.7 mg ropivacaine hydrochloride monohydrate, equivalent to 150 mg of ropivacaine hydrochloride. /…/ 10 mg/ml solution for injection: 1 ml contains 10.58 mg ropivacaine hydrochloride monohydrate, equivalent to 10 mg of ropivacaine hydrochloride. Excipient with known effect: sodium 2.9 mg/ml 10 ml amp. contains 105.8 mg ropivacaine hydrochloride monohydrate, equivalent to 100 mg of ropivacaine hydrochloride. 20 ml amp. contains 211.6 mg ropivacaine hydrochloride monohydrate, equivalent to 200 mg of ropivacaine hydrochloride. For thefull list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection Clear, colourless solution with a pH of 3.5 – 6 and an osmolality of 280 – 320 mosmol/kg. -

Biomedical Technology and Devices Handbook
1140_bookreps.fm Page 1 Tuesday, July 15, 2003 9:47 AM 29 Pharmaceutical Technical Background on Delivery Methods CONTENTS 29.1 Introduction 29.2 Central Nervous System: Drug Delivery Challenges to Delivery: The Blood Brain Barrier • Indirect Routes of Administration • Direct Routes of Administration 29.3 Cardiovascular System: Drug Delivery Chronotherapeutics • Grafts/Stents 29.4 Orthopedic: Drug Delivery Metabolic Bone Diseases 29.5 Muscular System: Drug Delivery 29.6 Sensory: Drug Delivery Ultrasound • Iontophoresis/Electroporation 29.7 Digestive System: Drug Delivery GI Stents • Colonic Drug Delivery 29.8 Pulmonary: Drug Delivery Indirect Routes of Administration • Direct Routes of Robert S. Litman Administration Nova Southeastern University 29.9 Ear, Nose, and Throat: Drug Delivery Maria de la Cova The Ear • The Nose • The Throat 29.10 Lymphatic System: Drug Delivery Icel Gonzalez 29.11 Reproductive System: Drug Delivery University of Memphis Contraceptive Implants • Contraceptive Patch • Male Contraceptive Eduardo Lopez References 29.1 Introduction In the evolution of drug development and manufacturing, drug delivery systems have risen to the forefront in the latest of pharmaceutical advances. There are many new pharmacological entities discov- ered each year, each with its own unique mechanism of action. Each drug will demonstrate its own pharmacokinetic profile. This profile may be changed by altering the drug delivery system to the target site. In order for a drug to demonstrate its pharmacological activity it must be absorbed, transported to the appropriate tissue or target organ, penetrate to the responding subcellular structure, and elicit a response or change an ongoing process. The drug may be simultaneously or sequentially distributed to a variety of tissues, bound or stored, further metabolized to active or inactive products, and eventually © 2004 by CRC Press LLC 1140_bookreps.fm Page 2 Tuesday, July 15, 2003 9:47 AM 29-2 Biomedical Technology and Devices Handbook excreted from the body.