Messages from the FAO E-Mail Conference on 'Gmos in the Pipeline'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
U.S. V. Bayer AG and Monsanto Company Comment: the Sierra Club
ATTN: Kathleen S. O'Neill Chief, Transportation, Energy & Agriculture Section Antitrust Division United States Department of Justice 450 5th Street, NW, Suite 800 Washington, DC 20530 Petition in opposition to proposed U.S. v. Bayer AG and Monsanto Company settlement and merger: A merger of agrochemical giants Bayer and Monsanto would create the world's largest seed and pesticide maker. I am afraid this move will reduce competition, raise prices for consumers and farmers, and result in an unacceptable degree of control over the agricultural industry and our food supply. I am very concerned about pollinators and the increased risks to bees, butterflies and birds with the increase of Bayer's neonicotinoids. Both companies produce corn products engineered to imply the use of harmful pesticides they manufacture. The production of corn uses high amounts of nitrogen- based fertilizers and the excess sediment is contaminating our waterways, therefore I am deeply worried about increased corn production from this merger. The heavy nutrient runoff from corn is widely attributed to exacerbating the marine "Dead Zone" in the Gulf of Mexico, in which algal blooms create hypoxic conditions wherein oxygen concentration is in such low levels that marine life suffocates and dies. I urge the Department of Justice to do more prevent the Bayer-Monsanto seed and pesticide platform from growing too strong by stopping this merger. If this merger is allowed, it should require more pesticide and seed divestments in order to protect our agriculture and food supply. This merger is anti-competition, if it is approved it will fail to protect farmers, consumers and the environment by allowing further consolidation of the industrial agriculture sector. -

Controlling Pregnancy: Fred Lyman Adair and the Influence of Eugenics on the Development of Prenatal Care
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine January 2019 Controlling Pregnancy: Fred Lyman Adair And The nflueI nce Of Eugenics On The evelopmeD nt Of Prenatal Care Florence Hsiao Follow this and additional works at: https://elischolar.library.yale.edu/ymtdl Recommended Citation Hsiao, Florence, "Controlling Pregnancy: Fred Lyman Adair And The nflueI nce Of Eugenics On The eD velopment Of Prenatal Care" (2019). Yale Medicine Thesis Digital Library. 3504. https://elischolar.library.yale.edu/ymtdl/3504 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. Controlling Pregnancy: Fred Lyman Adair and the Influence of Eugenics on the Development of Prenatal Care A Thesis Submitted to the Yale University School of Medicine In Partial Fulfillment of the Requirements for the Degree of Doctor of Medicine By Florence Hsiao Class of 2019 Abstract This thesis examines the development of prenatal care in the United States in the early 1900s by focusing on the life and career of Fred Lyman Adair who, as an obstetrician and eugenicist, played a significant role in shaping prenatal care into what it is today. Although prenatal care was a product of infant welfare activists and public health officials, obstetricians like Adair who struggled to establish obstetrics as a legitimate specialty, saw an opportunity in prenatal care to pathologize pregnancy and elevate their specialty. -

THE CASE for FIRST AMENDMENT PROTECTION of BIOART in 2000
FREEDOM TO EXPRESS A BIOLOGICAL CONSTRUCT: THE CASE FOR FIRST AMENDMENT PROTECTION OF BIOART KEITH SYVERSON INTRODUCTION In 2000, contemporary artist Eduardo Kac made international headlines by partnering with scientists at the Institut National de la Recherche Agronomique (INRA), France where together they genetically engineered an albino rabbit to express an enhanced green fluorescent protein (GFP)1 so that the rabbit would glow green when exposed to blue light.2 The entire project from its conception to the final display was intended as an artistic experiment to challenge society’s views on animal experimentation and the selective breeding of pets.3 This type of work that merges art and biology is called “BioArt.” According to Eduardo Kac, BioArt “employs one or more of the following approaches: (1) the coaching of biomaterials into specific inert shapes or behaviors; (2) the unusual or subversive use of biotech tools and processes; (3) the invention or transformation of living organisms with or without social environmental integration."4 BioArt has arguably existed for centuries.5 For example, some commentators have described the primitive methods of brewing beer in the Old Kingdom as form of BioArt.6 Similarly, the artificial selection involved in agriculture has been viewed by some as a form of 1 Frances Stracey, Bio-art: The Ethics Behind the Aesthetics, 10 NATURE REVIEWS: MOLECULAR CELL BIOLOGY 496, 499 (2009). 2 Id. 3 Id. 4 Eduardo Kac, Art That Looks You in the Eye: Hybrids, Clones, Mutants, Synthetics, and Transgenics, in SIGNS OF LIFE 1, 18 (Eduardo Kac ed., MIT Press, 2007). 5 See Edgar DaSilva, Art, Biotechnology and the Culture of Peace, 7 ELECTRONIC JOURNAL OF BIOTECHNOLOGY 130, 131-32 (2004) (arguing that the brewing reliefs in Egypt during the Old Kingdom from 2650 BC are an example of BioArt). -

SUMMARY Sign Offv7
Syngenta Event GA21 Page 1 of 29 PART II: SUMMARY Application for import and use of genetically modified herbicide tolerant maize Event GA21 under Regulation (EC) No 1829/2003 PART II: SUMMARY Syngenta Event GA21 Page 2 of 29 PART II: SUMMARY A . GENERAL INFORMATION 1. Details of application a) Member State of application UK b) Application number Not available at the time of submission c) Name of the product (commercial and other names) Maize Event GA21 In the USA, GA21 is marketed under the product name Agrisure GT Advantage (http://www.nk-us.com/infosilo/seedguide/agrisure.asp) d) Date of acknowledgement of valid application Not available at the time of submission Syngenta Event GA21 Page 3 of 29 PART II: SUMMARY 2. Applicant a) Name of applicant Syngenta Seeds S.A.S on behalf of Syngenta Crop Protection AG, Basel b) Address of applicant Syngenta Seeds S.A.S. 12, chemin de l'Hobit BP 27 F-31790 Saint-Sauveur On behalf of Syngenta Crop Protection AG, Basel Switzerland and all affiliated companies Schwarzwaldallee 215 CH 4058 Basle Switzerland c) Name and address of the person established in the Community who is responsible for the placing in the market, whether it be the manufacturer, the importer or the distributor, if different from the applicant (Commission Decision 2004/204/EC Art 3(a)(ii)) Event GA21 maize will be imported and used as any other maize in the EU by operators currently involved in these processes. 3. Scope of the application x GM plants for food use x Food containing or consisting of GM plants xFood produced from GM plants or containing ingredients produced from GM plants xGM plants for feed use x Feed containing or consisting of GM plants x Feed produced from GM plants x Import and processing (Part C of Directive 2001/18/EC) o Seeds and plant propagating material for cultivation in Europe (Part C of Directive 2001/18/EC) Syngenta Event GA21 Page 4 of 29 PART II: SUMMARY 4. -

The Era of Corporate Consolidation and the End of Competition Bayer-Monsanto, Dow-Dupont, and Chemchina-Syngenta
Research Brief October 2018 The Era of Corporate Consolidation and the End of Competition Bayer-Monsanto, Dow-DuPont, and ChemChina-Syngenta DISRUPT ECOSYSTEM ACCLERATE MONOPOLY THE EFFECTS OF CORPORATE CONSOLIDATION UNDERMINE FOOD SECURITY HARM SMALL PRODUCERS HAASINSTITUTE.BERKELEY.EDU This publication is published by the Haas Institute for a Fair and Inclusive Society at UC Berkeley This research brief is part of the Haas Institute's Shahidi Project from the Global Justice Program. The Shahidi Project (Shahidi is a Swahili word meaning “witness”) intends to demystify the power structures and capacities of transnational food and agricultural corporations within our food system. To that end, researchers have developed a robust database focusing on ten of the largest food and agricultural corporations in the world. See more at haasinstitute.berkeley.edu/shahidi. About the Authors Copyeditor Support Elsadig Elsheikh is the director Marc Abizeid Special thanks to the Food of the Global Justice program and Farm Communications at the Haas Institute for a Infographics Fund, which provided the seed Fair and Inclusive Society at Samir Gambhir funding for the Shahidi project. the University of California- Berkeley, where he oversees Report Citation Contact the program’s projects and Elsadig Elsheikh and Hossein 460 Stephens Hall research on corporate power, Ayazi. “The Era of Corporate Berkeley, CA 94720-2330 food system, forced migration, Consolidation and The End of Tel 510-642-3326 human rights, Islamophobia, Competition: Bayer-Monsanto, haasinstitute.berkeley.edu structural marginality and Dow-DuPont, and ChemChina- inclusion, and trade and Syngenta.” Haas Institute for development. a Fair and Inclusive Society at the University of California, Hossein Ayazi, PhD, is a Berkeley, CA. -

Media Release Syngenta Group: Growth of Sustainability- Enabling
Media Release Syngenta Group: Growth of sustainability- enabling products and services drives record H1 2021 Syngenta Group’s focus on helping farmers adapt to climate change and be part of the solution is creating growth opportunities • H1 Group sales at $14.4 billion (+$2.8 billion), +24 percent year-on-year • Q2 Group sales of $7.4 billion (+$1.6 billion), +28 percent year-on-year • H1 EBITDA at $2.7 billion, +22 percent year-on-year • Q2 EBITDA at $1.2 billion, +25 percent year-on-year • First half performance shows strong demand from farmers for sustainable products and services • Growth driven by Group’s innovation in seeds and crop protection products that enable regenerative agricultural practices • The Modern Agriculture Platform (MAP), which provides farmers with access to market-leading products and services, more than tripled sales year-on-year • Syngenta biologicals sales, including Valagro, grew 27 percent in H1, strengthening the Group’s leading position in this high growth segment 26 August 2021, Basel / Switzerland Syngenta Group today reported strong financial results for the second quarter and first half ended June 30, 2021. Group sales in second quarter were $7.4 billion, up 28 percent versus Q2 2020 (+25 percent at CER). EBITDA increased in the second quarter 25 percent (+38 percent at CER) to $1.2 billion. Group sales for the first half of 2021 were $14.4 billion, up 24 percent year-on-year (+18 percent at CER). EBITDA for the first half of the year was $2.7 billion, 22 percent higher year-on-year (+25 percent at CER). -

An Overview: Innovation in Plant Breeding
An Overview: Innovation in Plant Breeding Modern plant breeding blends traditional ways of developing crops with the latest in science and technology to achieve improved crops – enabling more choices for both farmers and consumers, and producing crops that can better cope with evolving pests, diseases and a changing climate. The Basics What: The simplest definition of plant breeding is crossing two plants Most of the fruits, vegetables, and to produce offspring that share the best characteristics of the two grains that we eat today are the parent plants. Breeders make crosses then select which offspring to advance in the pipeline based on their desired characteristics. result of generations of plant breeding. About 5,000 years ago, Why: Even the earliest farmers understood that, in order to survive, watermelons were only they needed plant varieties specifically adapted to their environmental conditions and cultivated to produce the best foods to nourish their 2 inches in diameter livestock and communities. and had a bitter taste, vastly How: Through generations of research and discovery, plant breeding has advanced beyond selecting a parent plant simply based on its different from appearance. It now includes an in-depth understanding of plants’ the large, genetic makeup, enabling breeders to better predict which plants will sweet-tasting have the highest probability of success in the field and the grocery fruit we enjoy store before making a cross. today. The Background The earliest farmers were plant breeders who understood the value of identifying crops that showed beneficial characteristics to plant in future seasons. Later, they learned they could cross two plants to develop an even better plant. -

Annual Monitoring Report on the Cultivation of MON 810 in 2008
Annual Monitoring Report on the Cultivation of MON 810 in 2008 Czech Republic, Germany, Portugal, Slovakia, Poland, Romania and Spain Submitted by MONSANTO EUROPE S.A. Dept. Regulatory Affairs Avenue de Tervuren 270-272 Tervurenlaan 270-272 B-1150 Brussels BELGIUM VOLUME 1 OF 1 July 2009 Data protection. This application contains scientific data and other information which are protected in accordance with Art. 31 of Regulation (EC) No 1829/2003. © 2009 Monsanto Company. All Rights Reserved. This document is protected under copyright law. This document is for use only by the regulatory authority to which this has been submitted by Monsanto Company, and only in support of actions requested by Monsanto Company. Any other use of this material, without prior written consent of Monsanto Company, is strictly prohibited. By submitting this document, Monsanto Company does not grant any party or entity any right to license, or to use the information of intellectual property described in this document. EXECUTIVE SUMMARY In 2008, Bt maize was planted in the EU on 107,719 hectares across seven countries (James, 2008). As part of stewardship of the technology, industry has implemented an Insect Resistance Management (IRM) plan to proactively avoid and/or delay the potential development of pest resistance to the Cry protein, as well as a voluntary general surveillance monitoring program. The adherence to these stewardship measures in the context of the cultivation of MON 810 maize in Europe is detailed in the Annual Monitoring Report on the Cultivation of MON 810 in 2008. The planting of MON 810 in the 2008 season was accompanied by a rigorous IRM plan involving three main elements: refuge implementation, monitoring and farmer education. -

The Debate on the Golden Rice and Its Background
The Debate on the Golden Rice and its Background A Literature Review Klaus Ammann, [email protected] Dedicated to the inventor and relentless promoter of Golden Rice, Ingo Potrykus Judge GM crops on their properties, not the technique used to make them – and we can start saving lives Editorial help: Vivian Moses, Patrick and Michael Moore 20140615 references numbered with full text links Millions of children die worldwide every year, an untenable situation that is still worsening which needs immediate correction. According to the World Health Organization (1), an estimated 250 million preschool children are vitamin A deficient (= VAD) and it is likely that in VAD areas a substantial proportion of pregnant women are also affected in 2013. Earlier reports (2) make evident that the problems are still growing: (in 2004: 140 million preschool children and more than 7 million pregnant women were suffering from VAD) 2 Preface It is not the intention of the author for this literature compilation on Golden Rice to replace the two websites www.goldenrice.org and www.allowgoldenricenow.org, they contain major information pieces, are well organized and specific information is easy to access. Rather it is the aim here to pull together a set of publications related to the background of the Golden Rice debate. We often are confronted with all kinds of determined opinions about the Golden Rice, Bio-Fortification, Transgenic Plants, Traditional and Organic Agriculture etc. and it is the purpose of this summary to shed light to the background of opinions pro and contra the Golden Rice – on how and why those opinions grow and how they are unfortunately melting down into simplistic slogans. -

View Presentation
Merck & Co Eli Lilly Bristol-Myers Squibb Novo Nordisk Pfizer GlaxoSmithKline Takeda Sanofi Novartis AstraZeneca Roche Otsuka Johnson & Johnson Otsuka Abbott Teva1 Amgen Daiichi Sankyo Bayer Boehringer Ingelheim Merck & Co Eli Lilly Bristol-Myers Squibb Novo Nordisk Pfizer GlaxoSmithKline Takeda Sanofi Novartis AstraZeneca Roche Otsuka Johnson & Johnson Otsuka Abbott Teva Amgen Daiichi Sankyo Bayer Boehringer Ingelheim Merck & Co Eli Lilly Bristol-Myers Squibb Novo Nordisk Pfizer GlaxoSmithKline Takeda Sanofi Novartis AstraZeneca Roche Otsuka Johnson & Johnson Otsuka Abbott Teva Amgen Daiichi Sankyo Bayer Boehringer Ingelheim Merck & Co Eli Lilly Bristol-Myers Squibb Novo Nordisk Pfizer GlaxoSmithKline Takeda Sanofi Novartis AstraZeneca Roche Otsuka Johnson & Johnson Otsuka Abbott Teva Amgen Daiichi Sankyo Bayer Boehringer Ingelheim Merck & Co Eli Lilly Bristol-Myers Squibb Novo Nordisk Pfizer GlaxoSmithKline Takeda Sanofi Novartis AstraZeneca Roche Otsuka Johnson & Johnson Otsuka Abbott Teva Amgen Daiichi Sankyo Bayer Boehringer Ingelheim Merck & Co Eli Lilly Bristol-Myers Squibb Novo Nordisk Pfizer GlaxoSmithKline Takeda Sanofi Novartis AstraZeneca Roche Otsuka Johnson & Johnson Otsuka Abbott Teva Amgen Daiichi Sankyo Bayer Boehringer Ingelheim Merck & Co Eli Lilly Bristol-Myers Squibb Novo Nordisk Pfizer GlaxoSmithKline Takeda Sanofi Novartis AstraZeneca Roche Otsuka Johnson & Johnson Otsuka Abbott Teva Amgen Daiichi Sankyo Bayer Boehringer Ingelheim Merck & Co Eli Lilly Bristol-Myers Squibb Novo Nordisk Pfizer GlaxoSmithKline Takeda -
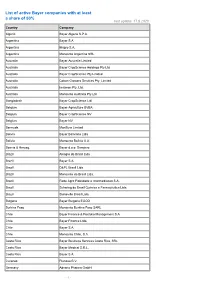
List of Active Bayer Companies with at Least a Share of 50% Last Update: 17.8.2020 Country Company
List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Algeria Bayer Algerie S.P.A. Argentina Bayer S.A. Argentina Biagro S.A. Argentina Monsanto Argentina SRL Australia Bayer Australia Limited Australia Bayer CropScience Holdings Pty Ltd Australia Bayer CropScience Pty Limited Australia Cotton Growers Services Pty. Limited Australia Imaxeon Pty. Ltd. Australia Monsanto Australia Pty Ltd Bangladesh Bayer CropScience Ltd. Belgium Bayer Agriculture BVBA Belgium Bayer CropScience NV Belgium Bayer NV Bermuda MonSure Limited Bolivia Bayer Boliviana Ltda Bolivia Monsanto Bolivia S.A. Bosnia & Herzeg. Bayer d.o.o. Sarajevo Brazil Alkagro do Brasil Ltda Brazil Bayer S.A. Brazil D&PL Brasil Ltda Brazil Monsanto do Brasil Ltda. Brazil Rede Agro Fidelidade e Intermediacao S.A. Brazil Schering do Brasil Química e Farmacêutica Ltda. Brazil Stoneville Brasil Ltda. Bulgaria Bayer Bulgaria EOOD Burkina Faso Monsanto Burkina Faso SARL Chile Bayer Finance & Portfolio Management S.A. Chile Bayer Finance Ltda. Chile Bayer S.A. Chile Monsanto Chile, S.A. Costa Rica Bayer Business Services Costa Rica, SRL Costa Rica Bayer Medical S.R.L. Costa Rica Bayer S.A. Curacao Pianosa B.V. Germany Adverio Pharma GmbH - 1 - List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Germany AgrEvo Verwaltungsgesellschaft mbH Germany Alcafleu Management GmbH & Co. KG Germany BGI Deutschland GmbH Germany Bayer 04 Immobilien GmbH Germany Bayer 04 Leverkusen Fußball GmbH Germany Bayer 04 Leverkusen -

Whither Plant Genetic Engineering? Allow Crops to Tolerate Environmental Stress Such As Drought, Cold, Salt, Heat, Or flood
PLANT TREK TO BOLDLY GO WHERE NO PLANT HAS GONE BEFORE On the Past, Present & Future of Plant Genetic Engineering by Richard G. Stout A HowPlantsWork.com eBook Copyright © 2013 by Richard G. Stout Version 1.0.1 PDF August, 2013 Table of Contents Preface Chapter 1: Where Do New Plants Come From? Chapter 2: How To Make A Transgenic Plant Chapter 3: Gene Guns, Terminators & Traitors Chapter 4: Farmaceuticals, Plantibodies & Edible Vaccines Chapter 5: Into The Wild Chapter 6: Are GM Plants Self-Replicating Inventions? Chapter 7: Plant Trek - The Next Generation Chapter 8: DIY Plant Genetic Engineering? Attributions About The Author Glossary about where plant biotechnology may be headed in the future, Preface including how plant biotechnology “hobbyists” may be getting into the act. Who is this book for? Please Note: This book is NOT a comprehensive textbook on plant genetic engineering and biotechnology. (If you’re looking This book is intended for people who may be curious about for such books, I’m sure you can find them at your local college plant genetic engineering, but who don’t want to read a long, bookstore or at an online bookseller.) Nor is this book meant to technical textbook on the subject. (There are provided, be a defense of genetically-engineered organisms (GMOs), however, ample links to books and articles - and also to online though I’m sure some readers will think so. Maybe here’s why. resources - for further reading.) If you’re looking for small “tastes” of information regarding various aspects of plant Since I was a graduate student in the 1970s at the University of genetic engineering, then this little book maybe just the Washington where some of the original work on transgenic informational “snack” that you’re looking for.