Investigating Genotype by Environment and QTL by Environment Interactions for Developmental Traits in Potato
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curriculum Vitae
CURRICULUM VITAE Name Ankit Patras Address 111 Agricultural and Biotechnology Building, Department of Agricultural and Environmental Sciences, Tennessee State University, Nashville TN 37209 Phone 615-963-6007, 615-963-6019/6018 Email [email protected], [email protected] EDUCATION 2005- 2009: Ph.D. Biosystems Engineering: School of Biosystems Engineering, College of Engineering & Architecture, Institute of Food and Health, University College Dublin, Ireland. 2005- 2006: Post-graduate certificate (Statistics & Computing): Department of Statistics and Actuarial Science, School of Mathematical Sciences, University College Dublin, Ireland 2003- 2004: Master of Science (Bioprocess Technology): UCD School of Biosystems Engineering, College of Engineering & Architecture, University College Dublin, Ireland 1998- 2002: Bachelor of Technology (Agricultural and Food Engineering): Allahabad Agriculture Institute, India ACADEMIC POSITIONS Assistant Professor, Food Biosciences: Department of Agricultural and Environmental Research, College of Agriculture, Human and Natural Sciences, Tennessee State University, Nashville, Tennessee 2nd Jan, 2014 - Present • Leading a team of scientist and graduate students in developing a world-class food research centre addressing current issues in human health, food safety specially virus, bacterial and mycotoxins contamination • Developing a world-class research program on improving safety of foods and pharmaceuticals • Develop cutting edge technologies (i.e. optical technologies, bioplasma, power Ultrasound, -

Talking Book Topics November-December 2016
Talking Book Topics November–December 2016 Volume 82, Number 6 About Talking Book Topics Talking Book Topics is published bimonthly in audio, large-print, and online formats and distributed at no cost to participants in the Library of Congress reading program for people who are blind or have a physical disability. An abridged version is distributed in braille. This periodical lists digital talking books and magazines available through a network of cooperating libraries and carries news of developments and activities in services to people who are blind, visually impaired, or cannot read standard print material because of an organic physical disability. The annotated list in this issue is limited to titles recently added to the national collection, which contains thousands of fiction and nonfiction titles, including bestsellers, classics, biographies, romance novels, mysteries, and how-to guides. Some books in Spanish are also available. To explore the wide range of books in the national collection, visit the NLS Union Catalog online at www.loc.gov/nls or contact your local cooperating library. Talking Book Topics is also available in large print from your local cooperating library and in downloadable audio files on the NLS Braille and Audio Reading Download (BARD) site at https://nlsbard.loc.gov. An abridged version is available to subscribers of Braille Book Review. Library of Congress, Washington 2016 Catalog Card Number 60-46157 ISSN 0039-9183 About BARD Most books and magazines listed in Talking Book Topics are available to eligible readers for download. To use BARD, contact your cooperating library or visit https://nlsbard.loc.gov for more information. -

Full-Text (PDF)
Vol. 13(6), pp. 153-162, June 2019 DOI: 10.5897/AJPS2019.1785 Article Number: E69234960993 ISSN 1996-0824 Copyright © 2019 Author(s) retain the copyright of this article African Journal of Plant Science http://www.academicjournals.org/AJPS Full Length Research Paper Adaptability and yield stability of bread wheat (Triticum aestivum) varieties studied using GGE-biplot analysis in the highland environments of South-western Ethiopia Leta Tulu1* and Addishiwot Wondimu2 1National Agricultural Biotechnology Research Centre, P. O. Box 249, Holeta, Ethiopia. 2Department of Plant Sciences, College of Agriculture and Veterinary Science, Ambo University. P. O. Box 19, Ambo, Ethiopia. Received 13 February, 2019; Accepted 11 April, 2019 The objectives of this study were to evaluate released Ethiopian bread wheat varieties for yield stability using the GGE biplot method and identify well adapted and high-yielding genotypes for the highland environments of South-western Ethiopia. Twenty five varieties were evaluated in a randomized complete block design with three replications at Dedo and Gomma during the main cropping season of 2016 and at Dedo, Bedelle, Gomma and Manna during the main cropping season of 2017, generating a total of six environments in location-by-year combinations. Combined analyses of variance for grain yield indicated highly significant (p<0.001) mean squares due to environments, genotypes and genotype-by- environment interaction. Yield data were also analyzed using the GGE (that is, G, genotype + GEI, genotype-by-environment interaction) biplot method. Environment explained 73.2% of the total sum of squares, and genotype and genotype X environment interaction explained 7.16 and 15.8%, correspondingly. -

March Wk01 Combined ARB
ILLINOIS WORKERS' COMPENSATION COMMISSION PAGE 1 C A S E H E A R I N G S Y S T E M MAUREEN PULIA 004 ARBITRATION CALL FOR QUINCY 42 004 ON 3/3/2021 SEQ CASE NBR PETITIONER NAME RESPONDENT NAME C O N N E C T E D C A S E S ACCIDENT PETITIONER ATTORNEY RESPONDENT ATTORNEY ↓ ↓ ↓ ↓ DATE ************************************************************************************ 1 03WC 03770 BUTLER, APRIL ADECCO EMPLOYMENT SERVICE 2/12/2011 THOMAS R LICHTEN NYHAN BAMBRICK KINZI 2 06WC 44900 TAYLOR, SHARON SOI, ILLINOIS VETERANS HO 4/9/2027 BERG & ROBESON ASSISTANT ATTORNEY G 3 08WC 23817 DORSEY, SHEILA ILLINI COMMUNITY HOSPITAL 8/1/2011 LAMARCA LAW OFFICES, PC NYHAN BAMBRICK KINZI 4 09WC 24313 STAPP, TROY RAIL CREW XPRESS 8/10/1930 THOMAS R LICHTEN 5 09WC 36074 WEMHOENER, SANDY K ILLINOIS VETERAN HOMES C O N N E C T E D C A S E S 7/4/2021 BERG & ROBESON ASSISTANT ATTORNEY G 09WC036075 12WC010594 14WC012250 6 09WC 49401 TAYLOR, SHARON ILLINOIS VETERANS HOME 5/7/2011 BERG & ROBESON ASSISTANT ATTORNEY G 7 10WC 45351 MEYER, WILLIAM G CCE C O N N E C T E D C A S E S 8/2/2028 THOMAS R LICHTEN BRADY, CONNOLLY & MA 10WC045352 10WC045353 8 12WC 22789 SIMMONS, RICHARD IDOT DISTRICT 6 12/4/2011 RIDGE & DOWNES LLC ASSISTANT ATTORNEY G 9 12WC 43805 CLARK, BRANDY LEE BLESSING HOSPITAL 12/9/2024 THOMAS R LICHTEN NYHAN BAMBRICK KINZI 10 13WC 04017 MORRISON, ANDREW C ILLINOIS VETERAN'S HOME C O N N E C T E D C A S E S 12/11/1930 GERALD TIMMERWILKE ASSISTANT ATTORNEY G 13WC004018 11 14WC 00548 WILLIS, CYNTHIA S NIEMANN FOODS INC KANOSKI BRESNEY SCHOLZ LOOS PALMER E 12 14WC 38789 SAXBERY, MARY A QUINCY PUBLIC SCHOOL DIST KATZ FRIEDMAN EAGLE ET AL BRYCE DOWNEY & LENKO 13 14WC 40062 BLENTLINGER-MARSHALL, NAN ST OF IL, QUINCY VETERANS CONNOR LAW OFFICES ASSISTANT ATTORNEY G 14 15WC 09029 MOON, CHANDLER DOT FOODS INC STEPHEN P. -

Multivariate Data Analysis in Sensory and Consumer Science
MULTIVARIATE DATA ANALYSIS IN SENSORY AND CONSUMER SCIENCE Garmt B. Dijksterhuis, Ph. D. ID-DLO, Institute for Animal Science and Health Food Science Department Lely stad The Netherlands FOOD & NUTRITION PRESS, INC. TRUMBULL, CONNECTICUT 06611 USA MULTIVARIATE DATA ANALYSIS IN SENSORY AND CONSUMER SCIENCE MULTIVARIATE DATA ANALYSIS IN SENSORY AND CONSUMER SCIENCE F NP PUBLICATIONS FOOD SCIENCE AND NUTRITIONIN Books MULTIVARIATE DATA ANALYSIS, G.B. Dijksterhuis NUTRACEUTICALS: DESIGNER FOODS 111, P.A. Lachance DESCRIPTIVE SENSORY ANALYSIS IN PRACTICE, M.C. Gacula, Jr. APPETITE FOR LIFE: AN AUTOBIOGRAPHY, S.A. Goldblith HACCP: MICROBIOLOGICAL SAFETY OF MEAT, J.J. Sheridan er al. OF MICROBES AND MOLECULES: FOOD TECHNOLOGY AT M.I.T., S.A. Goldblith MEAT PRESERVATION, R.G. Cassens S.C. PRESCOlT, PIONEER FOOD TECHNOLOGIST, S.A. Goldblith FOOD CONCEPTS AND PRODUCTS: JUST-IN-TIME DEVELOPMENT, H.R.Moskowitz MICROWAVE FOODS: NEW PRODUCT DEVELOPMENT, R.V. Decareau DESIGN AND ANALYSIS OF SENSORY OPTIMIZATION, M.C. Gacula, Jr. NUTRIENT ADDITIONS TO FOOD, J.C. Bauernfeind and P.A. Lachance NITRITE-CURED MEAT, R.G. Cassens POTENTIAL FOR NUTRITIONAL MODULATION OF AGING, D.K. Ingram ef al. CONTROLLEDlMODIFIED ATMOSPHERENACUUM PACKAGING, A. L. Brody NUTRITIONAL STATUS ASSESSMENT OF THE INDIVIDUAL, G.E. Livingston QUALITY ASSURANCE OF FOODS, J.E. Stauffer SCIENCE OF MEAT & MEAT PRODUCTS, 3RD ED., J.F. Price and B.S. Schweigert HANDBOOK OF FOOD COLORANT PATENTS, F.J. Francis ROLE OF CHEMISTRY IN PROCESSED FOODS, O.R. Fennema et al. NEW DIRECTIONS FOR PRODUCT TESTING OF FOODS, H.R. Moskowitz ENVIRONMENTAL ASPECTS OF CANCER: ROLE OF FOODS, E.L. Wynder et al. -
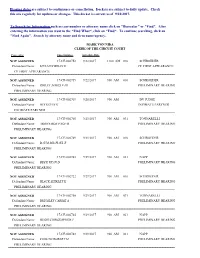
Hearing Dates Are Subject to Continuance Or Cancellation
Hearing dates are subject to continuance or cancellation. Dockets are subject to daily update. Check this site regularly for updates or changes. This docket is current as of 9/22/2017. To Search for Information such as case number or attorney name click on "Binocular" or "Find". After entering the information you want in the "Find What", click on "Find". To continue searching, click on "Find Again". Search by attorney name and firm name/agency. -

Relevance of Eating Pattern for Selection of Growing Pigs
RELEVANCE OF EATING PATTERN FOR SELECTION OF GROWING PIGS CENTRALE LANDB OUW CA TA LO GU S 0000 0489 0824 Promotoren: Dr. ir. E.W. Brascamp Hoogleraar in de Veefokkerij Dr. ir. M.W.A. Verstegen Buitengewoon hoogleraar op het vakgebied van de Veevoeding, in het bijzonder de voeding van de eenmagigen L.C.M, de Haer RELEVANCE OFEATIN G PATTERN FOR SELECTION OFGROWIN G PIGS Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen, op gezag van de rector magnificus, dr. H.C. van der Plas, in het openbaar te verdedigen op maandag 13 april 1992 des namiddags te vier uur in de aula van de Landbouwuniversiteit te Wageningen m st/eic/, u<*p' Cover design: L.J.A. de Haer BIBLIOTHEEK! LANDBOUWUNIVERSIIBtt WAGENINGEM De Haer, L.C.M., 1992. Relevance of eating pattern for selection of growing pigs (Belang van het voeropnamepatroon voor de selektie van groeiende varkens). In this thesis investigations were directed at the consequences of testing future breeding pigs in group housing, with individual feed intake recording. Subjects to be addressed were: the effect of housing system on feed intake pattern and performance, relationships between feed intake pattern and performance and genetic aspects of the feed intake pattern. Housing system significantly influenced feed intake pattern, digestibility of feed, growth rate and feed conversion. Through effects on level of activity and digestibility, frequency of eating and daily eating time were negatively related with efficiency of production. Meal size and rate of feed intake were positively related with growth rate and backfat thickness. -

General Linear Models - Part II
Introduction to General and Generalized Linear Models General Linear Models - part II Henrik Madsen Poul Thyregod Informatics and Mathematical Modelling Technical University of Denmark DK-2800 Kgs. Lyngby October 2010 Henrik Madsen Poul Thyregod (IMM-DTU) Chapman & Hall October 2010 1 / 32 Today Test for model reduction Type I/III SSQ Collinearity Inference on individual parameters Confidence intervals Prediction intervals Residual analysis Henrik Madsen Poul Thyregod (IMM-DTU) Chapman & Hall October 2010 2 / 32 Tests for model reduction Assume that a rather comprehensive model (a sufficient model) H1 has been formulated. Initial investigation has demonstrated that at least some of the terms in the model are needed to explain the variation in the response. The next step is to investigate whether the model may be reduced to a simpler model (corresponding to a smaller subspace),. That is we need to test whether all the terms are necessary. Henrik Madsen Poul Thyregod (IMM-DTU) Chapman & Hall October 2010 3 / 32 Successive testing, type I partition Sometimes the practical problem to be solved by itself suggests a chain of hypothesis, one being a sub-hypothesis of the other. In other cases, the statistician will establish the chain using the general rule that more complicated terms (e.g. interactions) should be removed before simpler terms. In the case of a classical GLM, such a chain of hypotheses n corresponds to a sequence of linear parameter-spaces, Ωi ⊂ R , one being a subspace of the other. n R ⊆ ΩM ⊂ ::: ⊂ Ω2 ⊂ Ω1 ⊂ R ; where Hi : µ 2 -

See Who Attended
Company Name First Name Last Name Job Title Country 24Sea Gert De Sitter Owner Belgium 2EN S.A. George Droukas Data analyst Greece 2EN S.A. Yannis Panourgias Managing Director Greece 3E Geert Palmers CEO Belgium 3E Baris Adiloglu Technical Manager Belgium 3E David Schillebeeckx Wind Analyst Belgium 3E Grégoire Leroy Product Manager Wind Resource Modelling Belgium 3E Rogelio Avendaño Reyes Regional Manager Belgium 3E Luc Dewilde Senior Business Developer Belgium 3E Luis Ferreira Wind Consultant Belgium 3E Grégory Ignace Senior Wind Consultant Belgium 3E Romain Willaime Sales Manager Belgium 3E Santiago Estrada Sales Team Manager Belgium 3E Thomas De Vylder Marketing & Communication Manager Belgium 4C Offshore Ltd. Tom Russell Press Coordinator United Kingdom 4C Offshore Ltd. Lauren Anderson United Kingdom 4Cast GmbH & Co. KG Horst Bidiak Senior Product Manager Germany 4Subsea Berit Scharff VP Offshore Wind Norway 8.2 Consulting AG Bruno Allain Président / CEO Germany 8.2 Consulting AG Antoine Ancelin Commercial employee Germany 8.2 Monitoring GmbH Bernd Hoering Managing Director Germany A Word About Wind Zoe Wicker Client Services Manager United Kingdom A Word About Wind Richard Heap Editor-in-Chief United Kingdom AAGES Antonio Esteban Garmendia Director - Business Development Spain ABB Sofia Sauvageot Global Account Executive France ABB Jesús Illana Account Manager Spain ABB Miguel Angel Sanchis Ferri Senior Product Manager Spain ABB Antoni Carrera Group Account Manager Spain ABB Luis andres Arismendi Gomez Segment Marketing Manager Spain -
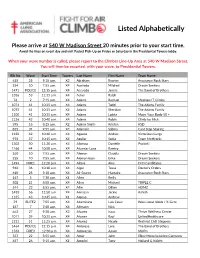
Listed Alphabetically
Listed Alphabetically Please arrive at 540 W Madison Street 20 minutes prior to your start time. Avoid the lines on event day and visit Packet Pick-Up on Friday or Saturday in the Presidential Towers lobby. When your wave number is called, please report to the Climber Line-Up Area at 540 W Madison Street. You will then be escorted, with your wave, to Presidential Towers. Bib No Wave Start Time Towers Last Name First Name Team Name 618 25 9:10 a.m. X2 Abraham Ryanne Assurance Rock Stars 254 10 7:55 a.m. X4 Acevedo Mildred Dream Seekers 1471 POLICE 12:15 p.m. X4 Acevedo Janete The Band of Brothers 1386 53 11:35 a.m. X4 Acker Ralph 76 2 7:15 a.m. X4 Adams Rachael Mesirow IT Climbs 1074 41 10:35 a.m. X4 Adams Todd The Adams Family 1075 41 10:35 a.m. X4 Adams Sheridan The Adams Family 1100 41 10:35 a.m. X4 Adams Loleta Move Your Body 50 + 1126 42 10:40 a.m. X4 Adams Robin Climb for Nick 395 16 8:25 a.m. X2 Adams Smith Kristen KSD 835 34 9:55 a.m. X4 Adamski Sabina Cant Stop Stairing 1120 42 10:40 a.m. X4 Aguero Andres Victorious Lungs 974 37 10:15 a.m. X4 Aguilar Sonia Team Medtronic 1302 50 11:20 a.m. X4 Albergo Danielle Protiviti 1166 44 10:50 a.m. X4 Alcantar Luna Ramiro 260 10 7:55 a.m. X4 Aleman Claudia Dream Seekers 255 10 7:55 a.m. -

Active-Warrants-02022018.Pdf
Last Name First Name Middle Name Date of Birth Warrant Type Violations ABBOTT PATRICK REYES 11/19/1993 MISD FTA ON SPEEDING ABDELLATIF FARES A 3/7/1982 MISD FTA-SPEEDING ABRAHAM CHRYN MARIE 7/22/1957 MISD FTOCO VAHCL CBO ABUDARAHIM MESHARI MOHAMMED 12/22/1992 MISD FTA-NO PROOF LIABILITY INS-ASP, SPEEDING ACHORD SHANNA BOUTTE 9/19/1982 MISD FTA-NO CHILD RESTRAINT ACOSTA AUGUSTIN SANTOS JR 9/24/1965 MISD FTA-SPEEDING, DWLS CBO ADAMS CALVIN DEMARK 1/5/1972 MISD FTOCOX2-DWI,IMPLIED CONSENT,POSS OF MARIJUANA, DISORDERLY CONDUCT ADAMS SHANNON RENE 3/30/1971 MISD FTOCOX2-NO DL ADAMS RAY CHARLES 6/14/1981 MISD FTOCO ON DWDLS ADANI ANTHONY JAMES 8/28/1984 MISD FTA SPEEDING CBO AGUILAR GABRIEL ABILA 2/28/1966 FEL FTOCO-CHILD SUPPORT CBO AGUILAR VICTOR RUIZ 7/23/1983 MISD FTA/NO DL CBO AGUILAR-GARZA JESUS E 11/2/1987 MISD FTA-NO DL, NO CHILD RESTRAINT AGUILAR-MARTINEZ GERMAN RAUL 12/2/1983 MISD FTA-NO DL, NO PROOF OF LIABILITY INS-ASP, NO SEAT BELT AGUILAR-TELLO WENDY JANET 12/5/1986 MISD FTA,NO DL, NO PROOF INS, CBO AHN JIN HWAN 8/12/1964 MISD FTA-NO DL, SPEEDING, NO PROOF OF LIABILITY INS-ASP CBO AIKIN AUSTIN KYLE 3/9/1992 MISD FTOCO,SPEEDING/DWLS AKIN DUSTIN KEITH 4/2/1984 CS FTOCO- CHILD SUPPORT CASH BOND AKIN GLENN ALLEN 9/7/1962 MISD FTOCO FTA NO INSURANCE NO DL AKINS PATRICA / / MISD FTA-FAILURE TO VACATE CBO ALANIZ CHELSEA MARIE 5/1/1989 MISD FTA-NO VEHICLE LICENSE (TAGS) CBO ALANIZ CHRISTOPHER LEE 12/25/1984 FEL PROB VIOL ON B&E, REVO X2 ALBARRAN CORIA JONATHAN 5/23/1985 MISD ftoco ALBERTY ASHLY SHANAYE 12/7/1991 MISD FTOCO,FTA,CURFEW VIOLATION,CBO -

Commencement Flyer
FPO UNIVERSITY OF MIAMI COMMENCEMENT2020 SPRING AND SUMMER UNIVERSITY OF MIAMI COMMENCEMENT2020 SPRING AND SUMMER Graduate School School of Architecture College of Arts and Sciences Patti and Allan Herbert Business School School of Communication School of Education and Human Development College of Engineering Rosenstiel School of Marine and Atmospheric Science Leonard M. Miller School of Medicine Phillip and Patricia Frost School of Music School of Nursing and Health Studies Message from the President JULIO FRENK To the Class of 2020, Congratulations! You are now prepared to undertake the next stage of your personal and professional development. Your education has equipped you with a unique skill set enriched by the experiences you have gained here at the University of Miami. The relationships you have forged with faculty, advisers, and fellow students have not only strengthened our community, but will undoubtedly be a source of community and strength throughout your career. I have always had an unwavering belief in your ability to persevere, but you have truly exhibited in an outstanding way the characteristics of caring and resilient ’Canes in the face of challenges such as hurricanes and now, the COVID-19 pandemic. This unprecedented emergency, which for the time being has transformed learning and upended life as we know it, forced the postponement of our spring commencement ceremonies until December. But that in no way affects your degree status, nor our immense pride in your accomplishments. I know you must feel frustration and disappointment at not being able to celebrate together at this time. If you choose to return in December, we will celebrate then.