Crystengcomm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carbon Dioxide Adsorption by Metal Organic Frameworks (Synthesis, Testing and Modeling)
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 8-8-2013 12:00 AM Carbon Dioxide Adsorption by Metal Organic Frameworks (Synthesis, Testing and Modeling) Rana Sabouni The University of Western Ontario Supervisor Prof. Sohrab Rohani The University of Western Ontario Graduate Program in Chemical and Biochemical Engineering A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © Rana Sabouni 2013 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Other Chemical Engineering Commons Recommended Citation Sabouni, Rana, "Carbon Dioxide Adsorption by Metal Organic Frameworks (Synthesis, Testing and Modeling)" (2013). Electronic Thesis and Dissertation Repository. 1472. https://ir.lib.uwo.ca/etd/1472 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. i CARBON DIOXIDE ADSORPTION BY METAL ORGANIC FRAMEWORKS (SYNTHESIS, TESTING AND MODELING) (Thesis format: Integrated Article) by Rana Sabouni Graduate Program in Chemical and Biochemical Engineering A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy The School of Graduate and Postdoctoral Studies The University of Western Ontario London, Ontario, Canada Rana Sabouni 2013 ABSTRACT It is essential to capture carbon dioxide from flue gas because it is considered one of the main causes of global warming. Several materials and various methods have been reported for the CO2 capturing including adsorption onto zeolites, porous membranes, and absorption in amine solutions. -

GERALDINE L. RICHMOND Website
1 GERALDINE L. RICHMOND Website: http://RichmondScience.uoregon.edu Address: 1253 University of Oregon, Eugene, OR 97403 Phone: (541) 346-4635 Email: [email protected] Fax: (541) 346-5859 EDUCATION 1976—1980 Ph.D. Chemistry, University of California, Berkeley, Advisor: George C. Pimentel 1971—1975 B.S. Chemistry, Kansas State University EMPLOYMENT 2013- Presidential Chair of Science and Professor of Chemistry, University of Oregon Research Interests: Understanding the molecular structure and dynamics of interfacial processes that have relevance to environmental remediation, biomolecular assembly, atmospheric chemistry and alternative energy sources. Teaching Interests: Science literacy for nonscientists; career development courses for emerging and career scientists and engineers in the US and developing countries. 2001-2013 Richard M. and Patricia H. Noyes Professor of Chemistry, University of Oregon 1998-2001 Knight Professor of Liberal Arts and Sciences, University of Oregon 1991- Professor of Chemistry, University of Oregon 1991-1995 Director, Chemical Physics Institute, University of Oregon 1985-l991 Associate Professor of Chemistry, University of Oregon 1980-1985 Assistant Professor of Chemistry, Bryn Mawr College AWARDS AND HONORS 2018 Priestley Medal, American Chemical Society 2017 Howard Vollum Award for Distinguished Achievement in Science and Technology, Reed College 2017 Honorary Doctorate Degree, Kansas State University 2017 Honorary Doctorate Degree, Illinois Institute of Technology 2016- Secretary, American Academy of Arts and Sciences; Member of the Board, Council and Trust 2015- U.S. State Department Science Envoy for the Lower Mekong River Countries 2015/16 President of the American Association for the Advancement of Science (AAAS) 2014 Pittsburgh Spectroscopy Award, Spectroscopy Society of Pittsburgh 2013 National Medal of Science 2013 Davisson-Germer Prize for Atomic or Surface Physics, American Physical Society 2013 Charles L. -

Crystengcomm
CrystEngComm View Article Online HIGHLIGHT View Journal | View Issue Metal–organic frameworks based luminescent materials for nitroaromatics sensing Cite this: CrystEngComm,2016,18, 193 Liangliang Zhang,† Zixi Kang,† Xuelian Xin and Daofeng Sun* Metal–organic frameworks (MOFs), composed of organic ligands and metal nodes, are well known for their high and permanent porosity, crystalline nature and versatile potential applications, which promoted them to be one of the most rapidly developing research focuses in chemical and materials science. During the Received 28th September 2015, various applications of MOFs, the photoluminescence properties of MOFs have received growing attention, Accepted 30th October 2015 especially for nitroaromatics (NACs) sensing, due to the consideration of homeland security, environmental cleaning and military issues. In this highlight, we summarize the progress in recent research in NACs sens- DOI: 10.1039/c5ce01917f ing based on LMOFs cataloged by sensing techniques in the past three years, and then we describe the www.rsc.org/crystengcomm sensing applications for nano-MOF type materials and MOF film, together with MOF film applications. Introduction 2,4-dinitrotoluene (2,4-DNT) and picric acid (PA), have become serious pollution sources of groundwater, soils, and With the increasing use of explosive materials in terrorism other security applications due to their explosivity and high all over the world, how to reliably and efficiently detect traces toxicity (Fig. 1). Detection of this class of explosives is -

Mysore University Library LIST of JOURNALS
Mysore University Library LIST OF JOURNALS SUBJECT: CHEMISTRY PRINT JOURNALS SUBSCRIBED Journal Name Current Science Indian Chemical Society Indian Journal of Chemical Technology Indian Journal of Chemistry Section - A Indian Journal of Chemistry Section - B Indian Journal of Heterocyclic Chemistry Journal of Applied Chemistry Journal of Applied Geochemistry Journal of Chemical Science Journal of Chemistry and Chemical Sciences Research Journal of Chemistry and Environment E-JOURNALS: UGC-INFONET & MUL PUBLISHERS/ E-JOURNALS URL AGGREGATOR Accounts of Chemical Research American Chemical Society http://pubs.acs.org/journals/achre4/index.html Acs chemical biology American Chemical Society http://pubs.acs.org/journals/acbcct/index.html Acta biomaterialia ScienceDirect http://www.sciencedirect.com/science/journal/17427061 Acta crystallographicasection a Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1600-5724 Acta crystallographica section b Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1600-5740 Acta crystallographica section c Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1600-5759 Acta crystallographica section d Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1399-0047 Acta crystallographica section e Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1600-5368 (electronic) Acta crystallographica section f Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1744-3091 (electronic) -

The Royal Society of Chemistry Turns Its Focus on Researchers with Better Search and Measurement Tools
The Royal Society of Chemistry turns its focus on researchers with better search and measurement tools The Royal Society of Chemistry offers a publishing platform providing access to over a million chemical science articles, book chapters and abstracts. Like many publishers of high quality peer-reviewed content, they are under pressure from their community to innovate quickly and harness digital technology in new ways that add value, simplicity and easier access to the research workflow. About Will Russell is responsible for some of the new technical developments • pubs.rsc.org at the Royal Society of Chemistry. “Although we do a lot of in-house • rsc.org development, we need to understand where developments can be • Location: Cambridge UK with improved by working with partners,” he says. “I really believe in the additional editorial teams in Beijing, benefit of strategic technology partnerships with an external partner. China, Bangalore India and There is the speed of getting a key utility to the market and this offers Washington D.C. USA us a tremendous business advantage.” • Scientific publisher of high-impact journals and books “We have journals going back to 1841,” he says. “We started migrating People print content online in the late 1990s. Our biggest challenge now is how • Will Russell we will deliver content in the future in the most useful way for the Business Relationship Manager researcher.” Goals Will pinpoints a way forward. “There are new opportunities presented • Embrace new technology to remain by open science and alternative metrics, and increasing importance competitive against innovative attached to data and open data,” he says. -

Winter for the Membership of the American Crystallographic Association, P.O
AMERICAN CRYSTALLOGRAPHIC ASSOCIATION NEWSLETTER Number 4 Winter 2004 ACA 2005 Transactions Symposium New Horizons in Structure Based Drug Discovery Table of Contents / President's Column Winter 2004 Table of Contents President's Column Presidentʼs Column ........................................................... 1-2 The fall ACA Council Guest Editoral: .................................................................2-3 meeting took place in early 2004 ACA Election Results ................................................ 4 November. At this time, News from Canada / Position Available .............................. 6 Council made a few deci- sions, based upon input ACA Committee Report / Web Watch ................................ 8 from the membership. First ACA 2004 Chicago .............................................9-29, 38-40 and foremost, many will Workshop Reports ...................................................... 9-12 be pleased to know that a Travel Award Winners / Commercial Exhibitors ...... 14-23 satisfactory venue for the McPherson Fankuchen Address ................................38-40 2006 summer meeting was News of Crystallographers ...........................................30-37 found. The meeting will be Awards: Janssen/Aminoff/Perutz ..............................30-33 held at the Sheraton Waikiki Obituaries: Blow/Alexander/McMurdie .................... 33-37 Hotel in Honolulu, July 22-27, 2005. Council is ACA Summer Schools / 2005 Etter Award ..................42-44 particularly appreciative of Database Update: -

Personal and Contact Details
CURRICULUM VITAE Carol Vivien Robinson DBE FRS FMedSci Personal and Contact Details Date of Birth 10th April 1956 Maiden Name Bradley Nationality British Contact details Department of Physical and Theoretical Chemistry University of Oxford South Parks Road Oxford OX1 3QZ Tel : +44 (0)1865 275473 E-mail : [email protected] Web : http://robinsonweb.chem.ox.ac.uk/Default.aspx Education and Appointments 2009 Professorial Fellow, Exeter College, Oxford 2009 Dr Lee’s Professor of Physical and Theoretical Chemistry, University of Oxford 2006 - 2016 Royal Society Research Professorship 2003 - 2009 Senior Research Fellow, Churchill College, University of Cambridge 2001 - 2009 Professor of Mass Spectrometry, Dept. of Chemistry, University of Cambridge 1999 - 2001 Titular Professor, University of Oxford 1998 - 2001 Research Fellow, Wolfson College, Oxford 1995 - 2001 Royal Society University Research Fellow, University of Oxford 1991 - 1995 Postdoctoral Research Fellow, University of Oxford. Supervisor: Prof. C. M. Dobson FRS 1991 - 1991 Postgraduate Diploma in Information Technology, University of Keele 1983 - 1991 Career break: birth of three children 1982 - 1983 MRC Training Fellowship, University of Bristol Medical School 1980 - 1982 Doctor of Philosophy, University of Cambridge. Supervisor: Prof. D. H. Williams FRS 1979 - 1980 Master of Science, University of Wales. Supervisor: Prof. J. H. Beynon FRS 1976 - 1979 Graduate of the Royal Society of Chemistry, Medway College of Technology, Kent 1972 - 1976 ONC and HNC in Chemistry, Canterbury -

New Journal and Database Subscriptions – 2012 -2013
NEW JOURNAL AND DATABASE SUBSCRIPTIONS – 2012 -2013 New Journals Afterall: A Journal of Art, Context and Enquiry American Biology Teacher American Journal of Bioethics American Political Thought Annals of Tourism Research Art Documentation Biodiversity and Conservation Biomaterials Science BioScience Boom: A Journal of California California Archaeology California Management Review Catalysis Science & Technology Chemical Hazards in Industry China Journal Classical Antiquity Classical Philology Crime and Justice Critical Review of International Social and Political Philosophy Education in Chemistry Educational Technology Research Development Elephant Ethics Federal Sentencing Reporter Food & Function Frankie Gastronomica: The Journal of Food and Culture Haaretz Historical Studies in the Natural Sciences HOPOS: The Journal of the International Society for the History of Philosophy of Science Huntington Library Quarterly Indian Country Today Indonesia Journal Information, Communication & Society Innovation Policy and the Economy Integrative Biology Issues in Environmental Science and Technology Journal of Applied Remote Sensing Journal of Digital Media Management Journal of Empirical Research on Human Research Ethics Journal of Environmental Studies and Sciences Journal of Human Capital Journal of Labor Economics Journal of Leisure Research Journal of Micro/Nanolithography, MEMS, and MOEMS Journal of Modern History Journal of Nanophotonics Journal of North African Studies Journal of Palestine Studies Journal of Photonics for Energy Journal -

RSC Gold 2015 Flyer.Pdf
RSC Gold Want access to full content from the world’s leading chemistry society? Including regular new material and an Archive dating back to 1841? Caltech’s RSC Gold Plus voucher codes to publish package subscription has been a very Open Access (OA) free of charge? welcome development ... I am very appreciative of the RSC Gold is the Royal Society of Chemistry’s general excellence of articles in the RSC premium package comprising 41 international research journals, evidenced by strong journals, literature updating services and impact factors and magazines that will meet the needs of all your increases in local download statistics. end-users. And the accompanying Gold for Gold Dana L. Roth OA voucher codes ensure maximum visibility for Chemistry Librarian your institution’s quality research. Caltech, USA Take a look inside to see exactly what you get www.rsc.org/gold RSC Gold includes a wealth of quality RSC journal, database and magazine content that is all available online. Journals Natural Product Reports Analyst New Journal of Chemistry Analytical Methods Organic & Biomolecular Chemistry Biomaterials Science Photochemical & Photobiological Sciences Catalysis Science & Technology Physical Chemistry Chemical Physics (PCCP) Chemical Communications Polymer Chemistry Chemical Science* RSC Advances Chemical Society Reviews Soft Matter CrystEngComm Toxicology Research Dalton Transactions Energy & Environmental Science B a c k fi l e Environmental Science: Nano** RSC Journals Archive 1841-2007 lease Environmental Science: Processes & Impacts -

Publishing Price List 2016
Publishing Price List 2016 Royal Society of Chemistry Collections for 2016 RSC GOLD INCLUDES: Key Royal Society of Chemistry online Price on application ONLINE ONLY† journal, database and magazine content, plus a EMAIL [email protected] book series. or contact your Account Manager PRICES JOURNALS ARCHIVE ONLINE ONLY† RSC Journals Archive Outright Purchase (1841 – 2007) • £41,097 • $72,615 RSC Journals Archive Outright Purchase (2005 – 2007) • £4,867 • $8,271 RSC Journals Archive Lease (1841 – 2007) • £7,408 • $13,133 PRICES RSC Journals Archive Hosting Fee • £819 • $1,340 THE HISTORICAL COLLECTION INCLUDES: Price on application ONLINE ONLY† • Society Publications (1949 – 2012) EMAIL [email protected] • Society Minutes (1841 – 1966) or contact your Account Manager • Historical Papers (1505 – 1991) PRICES CORE CHEMISTRY COLLECTION INCLUDES: • Chemical Communications • Dalton Transactions • Journal of Materials Chemistry A, B & C • New Journal of Chemistry PRINT & ONLINE† ONLINE ONLY† • Organic & Biomolecular Chemistry • • • Physical Chemistry Chemical Physics £19,685 £18,701 • RSC Advances (online only) • $36,814 • $34,973 PRICES GENERAL CHEMISTRY COLLECTION INCLUDES: • Chemical Communications • Chemical Society Reviews PRINT & ONLINE† ONLINE ONLY† • Chemistry World • £8,360 • £7,942 • New Journal of Chemistry PRICES • $13,685 • $13,244 • RSC Advances (online only) ANALYTICAL SCIENCE COLLECTION INCLUDES: • Analyst • Analytical Abstracts (online only) • Analytical Methods PRINT & ONLINE† ONLINE ONLY† • Environmental Science: Processes & Impacts • -

Journal Automatic Opt-In Unknown When Available After Acceptance
Journal Automatic Opt-In Unknown When Available After Acceptance American Chemical Society Accounts of Chemical Research X 30 minutes - 24 hours ACS Applied Materials & Interfaces X 30 minutes - 24 hours ACS Biomaterials Science & Engineering X 30 minutes - 24 hours ACS Catalysis X 30 minutes - 24 hours ACS Chemical Biology X 30 minutes - 24 hours ACS Chemical Neuroscience X 30 minutes - 24 hours ACS Combinatorial Science X 30 minutes - 24 hours ACS Infectious Diseases X 30 minutes - 24 hours ACS Macro Letters X 30 minutes - 24 hours ACS Medicinal Chemistry Letters X 30 minutes - 24 hours ACS Nano X 30 minutes - 24 hours ACS Photonics X 30 minutes - 24 hours ACS Sustainable Chemistry & Engineering X 30 minutes - 24 hours ACS Symposium Series X 30 minutes - 24 hours ACS Synthetic Biology X 30 minutes - 24 hours Advances in Chemistry X 30 minutes - 24 hours Analytical Chemistry X 30 minutes - 24 hours Biochemistry X 30 minutes - 24 hours Bioconjugate Chemistry X 30 minutes - 24 hours Biomacromolecules X 30 minutes - 24 hours Biotechnology Progress X 30 minutes - 24 hours Chemical & Engineering New Archives X 30 minutes - 24 hours C&EN Online X 30 minutes - 24 hours Chemical Research in Toxicology X 30 minutes - 24 hours Chemical Reviews X 30 minutes - 24 hours Chemistry of Materials X 30 minutes - 24 hours Inorganic Chemistry X 30 minutes - 24 hours Journal of the American Chemical Society X 30 minutes - 24 hours Journal of Chemical & Engineering Data X 30 minutes - 24 hours Journal of Chemical Information & Modeling X 30 minutes - 24 hours -
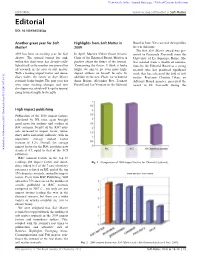
EDITORIAL | Soft Matter Editorial
View Article Online / Journal Homepage / Table of Contents for this issue EDITORIAL www.rsc.org/softmatter | Soft Matter Editorial DOI: 10.1039/b923355p Another great year for Soft Highlights from Soft Matter in Board in June. You can read their profiles Matter! 2009 later in this issue. The first Soft Matter award was pre- 2009 has been an exciting year for Soft In April, Martien Cohen Stuart became sented to Emanuela Zaccarelli from the Matter. The journal turned five and, Chair of the Editorial Board. Martien is University of La Sapienza, Rome. She within this short time, has already estab- positive about the future of the journal. was selected from a wealth of nomina- lished itself as the number one journal for ‘Concerning the future, I think it looks tions by the Editorial Board as a young all research in the area of soft matter. bright, we aim to get even more high- scientist who has produced significant With a leading impact factor and imme- impact authors on board!’ he says. In work that has advanced the field of soft diacy index, the future of Soft Matter addition to the new Chair, we welcomed matter. Professor Christos Likos, an certainly looks bright! The past year has Anna Balazs, Alejandro Rey, Lennart Advisory Board member, presented the seen some exciting changes and new Piculell and Jan Vermant to the Editorial award to Dr Zaccarelli during the developments, which will keep the journal going from strength to strength. High impact publishing Publication of the 2008 impact factors, calculated by ISI, once again brought good news for authors and readers of RSC journals.