Download This Article in PDF Format
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coastal and Marine Ecological Classification Standard (2012)
FGDC-STD-018-2012 Coastal and Marine Ecological Classification Standard Marine and Coastal Spatial Data Subcommittee Federal Geographic Data Committee June, 2012 Federal Geographic Data Committee FGDC-STD-018-2012 Coastal and Marine Ecological Classification Standard, June 2012 ______________________________________________________________________________________ CONTENTS PAGE 1. Introduction ..................................................................................................................... 1 1.1 Objectives ................................................................................................................ 1 1.2 Need ......................................................................................................................... 2 1.3 Scope ........................................................................................................................ 2 1.4 Application ............................................................................................................... 3 1.5 Relationship to Previous FGDC Standards .............................................................. 4 1.6 Development Procedures ......................................................................................... 5 1.7 Guiding Principles ................................................................................................... 7 1.7.1 Build a Scientifically Sound Ecological Classification .................................... 7 1.7.2 Meet the Needs of a Wide Range of Users ...................................................... -

Ctenophore Relationships and Their Placement As the Sister Group to All Other Animals
ARTICLES DOI: 10.1038/s41559-017-0331-3 Ctenophore relationships and their placement as the sister group to all other animals Nathan V. Whelan 1,2*, Kevin M. Kocot3, Tatiana P. Moroz4, Krishanu Mukherjee4, Peter Williams4, Gustav Paulay5, Leonid L. Moroz 4,6* and Kenneth M. Halanych 1* Ctenophora, comprising approximately 200 described species, is an important lineage for understanding metazoan evolution and is of great ecological and economic importance. Ctenophore diversity includes species with unique colloblasts used for prey capture, smooth and striated muscles, benthic and pelagic lifestyles, and locomotion with ciliated paddles or muscular propul- sion. However, the ancestral states of traits are debated and relationships among many lineages are unresolved. Here, using 27 newly sequenced ctenophore transcriptomes, publicly available data and methods to control systematic error, we establish the placement of Ctenophora as the sister group to all other animals and refine the phylogenetic relationships within ctenophores. Molecular clock analyses suggest modern ctenophore diversity originated approximately 350 million years ago ± 88 million years, conflicting with previous hypotheses, which suggest it originated approximately 65 million years ago. We recover Euplokamis dunlapae—a species with striated muscles—as the sister lineage to other sampled ctenophores. Ancestral state reconstruction shows that the most recent common ancestor of extant ctenophores was pelagic, possessed tentacles, was bio- luminescent and did not have separate sexes. Our results imply at least two transitions from a pelagic to benthic lifestyle within Ctenophora, suggesting that such transitions were more common in animal diversification than previously thought. tenophores, or comb jellies, have successfully colonized from species across most of the known phylogenetic diversity of nearly every marine environment and can be key species in Ctenophora. -

Features of Formation of Reefs and Macrobenthos Communities in the an Thoi Archipelago the Gulf of Thailand (South China Sea)
id7363687 pdfMachine by Broadgun Software - a great PDF writer! - a great PDF creator! - http://www.pdfmachine.com http://www.broadgun.com EEnnvviirroonnImSmSN : e0e97nn4 - 7tt45aa1 ll SSccViioleeumnne 8 Iccssuee 8 An Indian Journal Current Research Paper ESAIJ, 8(8), 2013 [297-307] Features of formation of reefs and macrobenthos communities in the An Thoi archipelago the Gulf of Thailand (South China Sea) Yuri Ya.Latypov A.V.Zhirmunsky Institute of Marine Biology, Far Eastern BranchRussian Academy of Sciences, Vladivostok, 690059, (RUSSIA) E-mail : [email protected] ABSTRACT KEYWORDS Macrobenthos communities studied on fringing reefs of the AnThoj Coral; archipelago using SCUBA-diving equipment. The islands are located in Reef; the turbid and highly eutrophic waters of the eastern Gulf of Thailand. We Macrobenthos; researched species composition and settlements densities and biomasses Community; in common species of algae, coelenterates, mollusks and echinoderms, as AnThoi archipelago; well as the degree of substrate coverage by macrophytes and coral. Clear Vietnam. vertical zonation identified in the change of the various communities in macrobenthos. The dominance of massive Porites on almost all reefs of the Gulf of Thailand is due to their ability to survive in stressful for many corals. They predominate over other scleractinian for the productivity of organic matter, the degree of substrate coverage and species diversity. They also constitute the reef skeleton and play a significant role of the expansion of its area in themuddy bottom of the Gulf of Thailand. 2013 Trade Science Inc. - INDIA INTRODUCTION phological zoning and developed powerful reef depos- its, common in structural reefs of the Indo- Pacific. -

An Analysis of the Fish and Macrobenthos Along the Sand Island Ocean Outfall Using Remote Video: Vi
HANAU-S-96-002 C2 AN ANALYSIS OF THE FISH AND MACROBENTHOS ALONG THE SAND ISLAND OCEAN OUTFALL USING REMOTE VIDEO: VI. 1995 DATA Richard E. Brock PROJECT REPORT PR-96-06 March 1996 WATER RESOURCES RESEARCH CENTER UNIlVERSlTY OF HAWAI'I AT MANOA Honolulu, Hawai'i 96822 AUTHOR: Dr. Richard E. Brock AssociateResearcher and FisheriesSpecialist Sea Grant Extension Service Marine Science Building 204 University of Hawai'i at Mhtoa Honolulu, Hawai'i 96822 Tel.: 808/956-2859 FAX: 808/956-2858 $5.0O/copy Please make remittance in U.S. dollars fmm a U.S. bank or internationalmoney order to: ResearchCorporatha ot the Uaiverekyot Hawaii Mail to. Water Resources Research Center University of Hawai'i at Mmnoa 2540 Dole St., Holmes Hall 283 Honolulu, Hawai'i 96822 ~ U.S.A. Attn: Publications Of6ce NOTE: Pleaseindicate PR-9646 on checkor moneyorder for our reference. AN ANALYSIS OF THE FISH AND MACROBKNTHOS ALONG THE SAND ISLAND OCEAN OUTFALL USING REMOTE VIDEO: VI. 1995 DATA Richard E. Brock Project Report PR-9644 March 1996 PREPARED FOR Departmentof WastewaterManagement City and County of Honolulu Project Report for "The Assessmentof the Impact of OceanSewer Outfalls on the Marine Environment off Oahu, Hawaii" ProjectNo.: C39805 ProjectPeriod: 1 January1995-31 August 1996 Principal Investigator: RogerS. Fujioka WATER RESOURCES RESEARCH CENTER University of Hawai'i at Minoa Honolulu, Hawai'i 96822 Any opinions.findings, and conclusions or recommendationsexpressed in thispublication are those of the author and do not necessarilyreflect the view of the Water ResourcesResearch Center, ABSTRACT Becausethe diffuser of theSand Island Ocean Outfall lies below safe diving depths, a remotely controlled video camerasystem was used to determinethe statusof the fish and diurnallyexposed macrobenthos resident to thediffuser. -

Comparative Composition, Diversity and Trophic Ecology of Sediment Macrofauna at Vents, Seeps and Organic Falls
Review Comparative Composition, Diversity and Trophic Ecology of Sediment Macrofauna at Vents, Seeps and Organic Falls Angelo F. Bernardino1*, Lisa A. Levin2, Andrew R. Thurber3, Craig R. Smith4 1 Departamento de Oceanografia e Ecologia, Universidade Federal do Espı´rito Santo, Goiabeiras, Vito´ ria, Esp´ı rito Santo, Brazil, 2 Center for Marine Biodiversity and Conservation; Integrative Oceanography Division, Scripps Institution of Oceanography, La Jolla, California, United States of America,3 College of Earth, Ocean, and Atmospheric Sciences, Oregon State University, Corvallis, Oregon, United States of America,4 Department of Oceanography, School of Ocean and Earth Science and Technology, University of Hawaii, Honolulu, Hawaii, United States of America communities. Sulfide is toxic to most metazoan taxa [1,2], Abstract: Sediments associated with hydrothermal vent- although some sediment-dwelling taxa have adapted to conditions ing, methane seepage and large organic falls such as of low oxygen and appear capable of tolerating the presence of whale, wood and plant detritus create deep-sea networks sulfide. Due to high local production, metazoans in reducing of soft-sediment habitats fueled, at least in part, by the sediments in the deep sea are often released from the extreme food oxidation of reduced chemicals. Biological studies at limitation prevalent in the background community (e.g. [3]). deep-sea vents, seeps and organic falls have looked at Instead, chemical toxicity may drive infaunal community macrofaunal taxa, but there has yet to be a systematic comparison of the community-level attributes of sedi- structure. In this meta-analysis we ask which taxa are common ment macrobenthos in various reducing ecosystems. -

The Structuring Role of Microhabitat Type in Coral Degradation Zones: a Case Study with Marine Nematodes from Kenya and Zanzibar
Coral Reefs (2007) 26:113–126 DOI 10.1007/s00338-006-0184-8 REPORT The structuring role of microhabitat type in coral degradation zones: a case study with marine nematodes from Kenya and Zanzibar M. Raes · M. De Troch · S. G. M. Ndaro · A. Muthumbi · K. Guilini · A. Vanreusel Received: 31 July 2006 / Accepted: 20 November 2006 / Published online: 7 February 2007 © Springer-Verlag 2007 Abstract Nematode genus assemblages were identi- three-dimensional structure of coral fragments was Wed from four locations in coral degradation zones found. DiVerences between nematode assemblages in (CDZs) along the African east coast: Watamu and the coralline sediment and on coral fragments were Tiwi Beach (Kenya) and Matemwe and Makunduchi attributed to the exposed nature of the latter habitat, (Zanzibar). Three microhabitat types were distin- its large surface area and its microbial or algal cover. guished: coralline sediment, coral gravel and coral frag- DiVerences in available food sources were reXected in ments. Nematode community composition was nematode trophic composition. comparable to that of other studies dealing with the same habitat. The presence of a common genus pool in Keywords Coral degradation zones · Nematodes · CDZs was reXected in the considerable similarities Microhabitats · Spatial turnover · Indian Ocean between samples. The addition of coral fragments as a habitat for nematodes resulted in an increased impor- tance of taxa typical for coarse sediments and large Introduction substrata. Local and regional turnover were of the same order of magnitude. The structuring eVect of Numerous studies have investigated the major factors microhabitat type clearly overrode the eVect on a local that structure nematode community composition in and regional scale. -

Articles and Detrital Matter
Biogeosciences, 7, 2851–2899, 2010 www.biogeosciences.net/7/2851/2010/ Biogeosciences doi:10.5194/bg-7-2851-2010 © Author(s) 2010. CC Attribution 3.0 License. Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem E. Ramirez-Llodra1, A. Brandt2, R. Danovaro3, B. De Mol4, E. Escobar5, C. R. German6, L. A. Levin7, P. Martinez Arbizu8, L. Menot9, P. Buhl-Mortensen10, B. E. Narayanaswamy11, C. R. Smith12, D. P. Tittensor13, P. A. Tyler14, A. Vanreusel15, and M. Vecchione16 1Institut de Ciencies` del Mar, CSIC. Passeig Mar´ıtim de la Barceloneta 37-49, 08003 Barcelona, Spain 2Biocentrum Grindel and Zoological Museum, Martin-Luther-King-Platz 3, 20146 Hamburg, Germany 3Department of Marine Sciences, Polytechnic University of Marche, Via Brecce Bianche, 60131 Ancona, Italy 4GRC Geociencies` Marines, Parc Cient´ıfic de Barcelona, Universitat de Barcelona, Adolf Florensa 8, 08028 Barcelona, Spain 5Universidad Nacional Autonoma´ de Mexico,´ Instituto de Ciencias del Mar y Limnolog´ıa, A.P. 70-305 Ciudad Universitaria, 04510 Mexico,` Mexico´ 6Woods Hole Oceanographic Institution, MS #24, Woods Hole, MA 02543, USA 7Integrative Oceanography Division, Scripps Institution of Oceanography, La Jolla, CA 92093-0218, USA 8Deutsches Zentrum fur¨ Marine Biodiversitatsforschung,¨ Sudstrand¨ 44, 26382 Wilhelmshaven, Germany 9Ifremer Brest, DEEP/LEP, BP 70, 29280 Plouzane, France 10Institute of Marine Research, P.O. Box 1870, Nordnes, 5817 Bergen, Norway 11Scottish Association for Marine Science, Scottish Marine Institute, Oban, -

Sieve Size Influence in Estimating Biomass, Abundance and Diversity in Samples of Deep-Sea Macrobenthos
MARINE ECOLOGY PROGRESS SERIES Vol. 225: 97–107, 2002 Published January 11 Mar Ecol Prog Ser Sieve size influence in estimating biomass, abundance and diversity in samples of deep-sea macrobenthos John D. Gage*, David J. Hughes, José L. Gonzalez Vecino Scottish Association for Marine Science, Dunstaffnage Marine Laboratory, Oban PA34 4AD, United Kingdom ABSTRACT: A divergence in sieve size protocols for washing samples has arisen among shallow- and deep-sea benthic biologists, which now affects comparability across the 2 environments. This has come about as a result of a perception of smaller body size among deep-sea benthic organisms. Two box-core samples from ~1900 m depth were examined to see how different sieve size affects estima- tion of biomass, abundance and diversity of macrofauna in the deep sea. Expressed as cumulative retentions, the coarsest sieve (1 mm mesh) retained 94% of the biomass retained in the finest sieve (0.25 mm), confirming the expectation that rarer, large-sized individuals contribute most to biomass of macrobenthos. There were small increases when progressively finer sieves were used, but the rate of increase declined markedly from 0.425 to 0.25 mm mesh. Numbers of individual organisms increased through the series, with a marked increase from 0.5 to 0.425 mm mesh; numbers of species did not increase as rapidly, but showed a similarly high rate between 0.5 and 0.425 mm mesh. The proportions of the total biomass in coarser sieves, and the total number of specimens retained by the finest sieve were rather similar to those shown in previous studies of inshore and continental shelf benthos. -

General Bait Rules Match Fishing Rules
Barford Lakes, Chapel Street, Barford, Norwich, Norfolk, NR9 4BJ Tel: 01603 759624 Fax: 01603 758111 email: [email protected] www.barfordlakes.com www.barfordtackle.com Match Fishing Rules - ALL LAKES Manufactured Barbless hooks only - max hook size standard size 8 or equivalent Anglers to lay their keepnets and landing net out behind their peg on arrival. Keepnets to enter lake 15 minutes before the start of the match. Lifting and dropping allowed but no suspending of bait other than by a float No surface fishing Leads and feeders free running to a bead. A sliding float stop 12” from your feeder is allowed. Method feeders - inline and flat bed only - no elasticated feeders except Guru X Safe System Method Feeder are allowed. 4” minimum hook length for feeder or ledger No Floating pole method - line between pole tip to top of float - minimum of 6” and maximum of 1metre Minimum 12” of line between bottom of float and hook. When fishing within 1 metre of bankside vegetation no line limit applies No bubble or feeder floats of any type You can not feed with a pole whilst fishing a rod No hand-lining – unless necessary i.e. broken top section, foul hooked fish (pulla kits/bungs permitted) Bait Rules No Peanuts ● Groundbait allowed - 3 kilo limit Luncheon meat - 2 small tins one 340grm tin allowed Liquidised & riddled baits not to exceed 1 kilo or a 3.3 pint bait box Barford Lakes carp feed pellets only Paste, hook/banded/expanda pellets & boilies are allowed for HOOK BAIT ONLY. 0. Boilie & Pellet Hook Baits to be stored in small tub (no larger than a 1 pint bait box) All feed baits to be used in moderation • NO BAIT to enter the water after the match - please take it home or use the bin provided. -

Assessment of Macrobenthos Response to Sediment Contamination in the San Francisco Estuary, California, Usa
Environmental Toxicology and Chemistry, Vol. 23, No. 9, pp. 2178±2187, 2004 q 2004 SETAC Printed in the USA 0730-7268/04 $12.00 1 .00 ASSESSMENT OF MACROBENTHOS RESPONSE TO SEDIMENT CONTAMINATION IN THE SAN FRANCISCO ESTUARY, CALIFORNIA, USA BRUCE THOMPSON* and SARAH LOWE San Francisco Estuary Institute, 7770 Pardee Lane, Oakland, California 94621, USA (Received 30 April 2003; Accepted 18 February 2004) AbstractÐA multimetric benthic assessment method was developed for two benthic assemblages in the San Francisco Estuary (USA) using data from several monitoring programs collected over ®ve years. Assessment indicators used were total number of taxa, total abundances, oligochaete abundances, number of molluscan taxa, number of amphipod taxa, and Capitella capitata and Streblospio benedicti abundances. Exceedances of the maximum or minimum indicator values in reference samples were used to assess test samples using a weight-of-evidence to obtain an assessment value. Only 2.5% of the samples from the deeper, offshore sites had benthic impacts, 14.3% of the samples from near wastewater discharges had impacts, and 78.3% of the samples from the estuary margins and channels were impacted. Impacted samples from both assemblages had signi®cantly higher mean effects range- median quotient values (mERMq) than reference samples, total organic carbon (TOC) was signi®cantly higher in the impacted samples from the mesohaline assemblage, and percent ®nes was signi®cantly higher in the impacted samples from the polyhaline assemblage, re¯ecting the close associations of contaminants with ®ne sediments and organic material. In samples with mERMq below 0.050, there were no benthic impacts. The incidence of impacts remained low (9.4%) at mERMq below 0.146, but when mERMq was above 0.146, 68.2% of the samples had benthic impacts, and samples with mERMq above 0.740 were always impacted. -

Status of Eutrophication in San Elijo Lagoon and Its Relevance for Restoration
Status of Eutrophication in San Elijo Lagoon and its Relevance for Restoration Martha Sutula, David J. Gillett, and Aaron Jones Southern California Coastal Water Research Project July 2016 Technical Report 938 EXECUTIVE SUMMARY San Elijo Lagoon (the Lagoon) in San Diego County, California, is undergoing restoration planning to ameliorate problems associated with human impacts on hydrology, habitat and water quality. Increased watershed and nutrient runoff, legacy sewage disposal, and restricted tidal exchange have caused a buildup of organic matter in Lagoon sediments. This accumulated organic matter, otherwise known as eutrophication, causes nuisance algal blooms, consumes dissolved oxygen (DO), causing hypoxia and related fish kills, and degradation of benthic habitat that provides important forage for estuarine fish, resident and migratory birds. Collectively, these symptoms of eutrophication have caused the Lagoon to be placed on the 303(d) list of impaired waterbodies. The San Elijo Lagoon Restoration Project (SELRP) was proposed to improve ecological function and services of the Lagoon by two primary means: 1) reconfiguring lagoon elevations via grading/dredging to support specific habitat types and to remove legacy sediment organic matter from sewage disposal sites and 2) modifying water flow in the Lagoon via changes to the ocean inlet and lagoon channels. As the SELRP nears completion of the restoration permitting process, additional documentation of the status of eutrophication symptoms in the Lagoon is desired by SELC in order to support decision-making on the restoration by SELRP lead agencies. The goal of this report is to summarize the status of eutrophication symptoms in the Lagoon, utilizing existing data collected through SELC, Bight Regional, and County monitoring programs and other available special studies (2003 – 2016). -
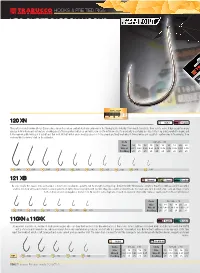
Xps Supercarbon Hooks
HOOKS & PRE TIED RIGS XPS SUPERCARBON HOOKS Extra Performance Series is the name of Trabucco quality ho- oks developed for top match anglers, which now gets much wider also touching different segments such as feeder fishing, commercial carping, trout fishing, light drifting and beach ledgering. In line with existing series, also the new ones display “extra performance”, because the extreme quality is not only achieved with the development of new shapes, but also through the em- ployed materials, the finish and functional details. The strength/ lightness ratio has been tailored size by size, taking advantage of the special hi-carbon content steel, which is only available in Japan, where these fantastic hooks are produced under strict quality control. All points are chemically sharpened, then further finished by laser to get absolute perfection and durability. 5($/6,=(( 120 XN N Nickel Regular This pattern is well known all over Europe since when the carbon content steel was unknown in the fishing tackle industry. From roach to mullets, from eels to soles, it has caught so many species both in fresh and salt waters, showing great efficiency when baited up and while removed from fish mouth. It’s popularity is certainly also due to the long point, parallel to shank, and to the narrow ankle which provide bait and fish hold. All bait which are normally pushed on to the shank are finely matching it: from pinkies and squatts to earthworms in freshwater; from enchovy fillet to shrimp’s tail in the saltwater. &RGH 6L]H :LUH 3FV%DJ 121 XB BR Bronzed Forged MicroBarb Its code recalls the classic 120, as its shape comes from long shank popularity, but its strength is stepped up.