AMM 2021-2 Na Web AMM 21-2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Malignant Hidradenoma: a Report of Two Cases and Review of the Literature
ANTICANCER RESEARCH 26: 2217-2220 (2006) Malignant Hidradenoma: A Report of Two Cases and Review of the Literature I.E. LIAPAKIS1, D.P. KORKOLIS2, A. KOUTSOUMBI3, A. FIDA3, G. KOKKALIS1 and P.P. VASSILOPOULOS2 1Department of Plastic and Reconstructive Surgery, 2First Department of Surgical Oncology and 3Department of Surgical Pathology, Hellenic Anticancer Institute, "Saint Savvas" Hospital, Athens, Greece Abstract. Introduction: Malignant tumors of the sweat glands difficult (1). Clear cell hidradenoma is an extremely rare are very rare. Clear cell hidradenoma is a lesion with tumor with less than 50 cases reported (2, 3). histopathological features resembling those of eccrine poroma The cases of two patients, suffering from aggressive and eccrine spiradenoma. The biological behavior of the tumor dermal lesions invading the abdominal wall and the axillary is aggressive, with local recurrences reported in more than 50% region, are described here. Surgical resection and of the surgically-treated cases. Materials and Methods: Two histopathological examination ascertained the presence of patients are presented, the first with tumor in the right axillary malignant clear cell hidradenoma. In addition to these region, the second with a recurrent tumor of the abdominal cases, a review of the literature is also presented. wall. The first patient underwent wide excision with clear margins and axillary lymph node dissection and the second Case Reports patient underwent wide excision of the primary lesion and bilateral inguinal node dissection due to palpable nodes. Patient 1. Patient 1 was a 68-year-old Caucasian male who had Results: The patients had uneventful postoperative courses. No undergone excision of a rapidly growing, ulcerous lesion of the additional treatment was administered. -

University of Dundee Hidradenoma Masquerading Digital
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of Dundee Online Publications University of Dundee Hidradenoma masquerading digital ganglion cyst Makaram, Navnit; Chaudhry, Iskander H.; Srinivasan, Makaram S. Published in: Annals of Medicine and Surgery DOI: 10.1016/j.amsu.2016.07.017 Publication date: 2016 Document Version Publisher's PDF, also known as Version of record Link to publication in Discovery Research Portal Citation for published version (APA): Makaram, N., Chaudhry, I. H., & Srinivasan, M. S. (2016). Hidradenoma masquerading digital ganglion cyst: a rare phenomenon. Annals of Medicine and Surgery , 10, 22-26. DOI: 10.1016/j.amsu.2016.07.017 General rights Copyright and moral rights for the publications made accessible in Discovery Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from Discovery Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain. • You may freely distribute the URL identifying the publication in the public portal. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 17. Feb. 2017 Annals of Medicine and Surgery 10 (2016) 22e26 Contents lists available at ScienceDirect Annals of Medicine and Surgery journal homepage: www.annalsjournal.com Case report Hidradenoma masquerading digital ganglion cyst: A rare phenomenon * Navnit Makaram a, , Iskander H. -

Brooke-Spiegler Syndrome – an Underrecognized Cause of Multiple Familial Scalp Tumors: Report of a New Germline Mutation
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repositório Institucional dos Hospitais da Universidade de Coimbra 67 DOI: http://dx.doi.org/10.3315/jdcr.2015.1208 Brooke-Spiegler Syndrome – an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation André Castro Pinho, Miguel José Pinto Gouveia, Ana Rita Portelinha Gameiro, José Carlos Pereira Silva Cardoso, Maria Margaria Martins Gonçalo Dermatology Department of Centro Hospitalar e Universitário de Coimbra, Portugal. Corresponding author: Abstract André Castro Pinho, MD Background: Brooke-Spiegler syndrome (BSS) is probably an underdiagnosed ge- Dermatology Department nodermatosis that predisposes for the development of cylindromas, spiradeno- mas and trichoepitheliomas mainly of the head and neck. Wide phenotypic varia- Hospitais da Universidade de Coimbra bility regarding the number and type of lesions can be observed within a family. Centro Hospitalar e Universitário Mutations of the CYLD gene are identified in the vast majority of cases and play de Coimbra a key role in BSS pathogenesis. Praceta Mota Pinto, 3000-075 Coimbra, Main observations: Two first degree relatives with numerous erythematous te- Portugal langiectatic nodules of the scalp present for decades, with recurring tendency re- gardless the multiple previous excisions. Histopathological review of the lesions E-mail: [email protected] revealed predominantly "spiradenocylindromas" in the proband and cylindromas in her sister. The suspicion of BSS was confirmed after detection of a new non- sense germline mutation of CYLD (c.1783C>T pGln 595*) in the proband. Conclusions: BSS diagnosis can be challenging and is based on clinical-patholo- gical correlation, positive familial association and identification of CYLD mutations. -

Adnexal Tumors
10/24/2019 What’s a gland like you doing in a place like this? A practical approach to cutaneous adnexal neoplasms Hafeez Diwan, MD, PhD Departments of Pathology & Immunology and Dermatology Baylor College of Medicine 1 Conflict of interest • None 2 Disclosures • I have nothing to disclose 3 1 10/24/2019 Is the adnexal neoplasm glandular? And if so, where is it located? • Hands and Feet: Digital papillary adenocarcinoma 4 5 6 2 10/24/2019 7 8 Digital Papillary Adenocarcinoma • Solitary • Fingers/toes/palms/soles • Recurrence/metastases 9 3 10/24/2019 10 11 12 4 10/24/2019 3 Points about digital papillary adenocarcinoma • 1. Atypia doesn’t matter – if there is no atypia, it doesn’t mean that it isn’t digital papillary adenocarcinoma 13 3 Points about digital papillary adenocarcinoma • 1. Atypia doesn’t matter – if there is no atypia, it doesn’t mean that it isn’t digital papillary adenocarcinoma • 2. How high can the glandular lesion go up the extremity? • Example of one case that occurred on the thigh? (Alomari A, Douglas S, Galan A, Narayan D, Ko C. Atypical Presentation of digital papillary adenocarcinoma (abstract) J Cutan Pathol. 2014;41:221) 14 3 Points about digital papillary adenocarcinoma (cont’d) • 3. What if you don’t see glands • Hidradenoma on hands and feet • Hunt for a gland? If you see a gland, then what? • Probably best to err on the side of caution and say that a digital papillary adenocarcinoma is not ruled out 15 5 10/24/2019 16 17 18 6 10/24/2019 19 20 21 7 10/24/2019 3 Points about digital papillary adenocarcinoma (cont’d) • 3. -

Sebaceous Neoplasia: Diagnosis, Immunohistochemical Studies, Molecular-Genetics and Relationships to Muir-Torre Syndrome Michael T
Sebaceous neoplasia: Diagnosis, immunohistochemical studies, molecular-genetics and relationships to Muir-Torre Syndrome Michael T. Tetzlaff MD, PhD Associate Professor Departments of Pathology and Translational and Molecular Pathology The University of Texas MD Anderson Cancer Center Houston, Texas Executive Officer Translational Research Program The Alliance for Clinical Trials No relevant conflicts of interest to disclose. Advisory board with Seattle Genetics, Myriad Genetics, Novartis and Galderma. Sebaceous neoplasia • Histopathologic features of sebaceous gland proliferations • Sebaceous hyperplasia • Sebaceous adenoma • Sebaceous epithelioma • Sebaceous carcinoma • Sebaceous gland neoplasia and the relationships to DNA mismatch repair • Molecular genetic alterations of sebaceous carcinoma: • Differentially expressed miRNAs • Mutational signature in sebaceous carcinoma Normal sebaceous glands Normal sebaceous gland Sebaceous hyperplasia • Clinically small, flesh colored papules—typically with a central depression or umbilication on the face • Benign overabundance of superficial sebaceous lobules surrounding a centrally dilated follicular structure normal- appearing sebaceous lobules • Sebaceous lobules are increased in number and abut epidermis • Sebocytes predominate over basaloid cells (only 1-2 layers) Sebaceous hyperplasia Not related to defects in mismatch repair The spectrum of sebaceous neoplasia • Sebaceous adenoma • Sebaceous epithelioma/sebaceoma • Sebaceous carcinoma Sebaceous Adenoma • Benign proliferation of sebocytes -

Malignant Eccrine Acrospiroma with Nodal and Bone Metastasis
Case Report Malignant eccrine acrospiroma with nodal and bone metastasis Burhan Wani, Shiekh Aejaz Aziz, Mohmad Hussain Mir, Gull Mohammad Bhat, Abdul Rashid Lone Department of Medical Oncology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar 190011, Kashmir, India. Correspondence to: Dr. Burhan Wani, Department of Medical Oncology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar 190011, Kashmir, India. E-mail: [email protected] ABSTRACT Acrospiromas are cutaneous tumors of sweat duct differentiation. Although various eccrine sweat gland tumours including benign acrospiroma are widely reviewed, malignant acrospiroma is rarely reported. Clinically, they resemble other cutaneous lesions and the primary treatment is wide local excision with or without lymph node dissection. The efficacy of adjuvant chemotherapy and radiation therapy requires further investigation. Key words: Acrospiroma; metastasis; chemotherapy; radiotherapy INTRODUCTION right inguinal region. The swelling was firm in consistency and mildly tender. There was another mass 2 cm below Acrospiroma represents a group of benign ductal tumors this measuring 3 cm × 2 cm, firm in consistency, mobile, of the eccrine sweat glands that sometimes are connected non-tender with normal overlying skin, felt to be a lymph to the skin, ranging from solitary plaques to exophytic node clinically. papules or dermal nodules.[1] Malignant acrospiroma (Syn: malignant nodular/clear cell hidradenoma, malignant clear The patient was operated on and excision of the mass cell acrospiroma, clear cell eccrine carcinoma, primary along with inguinal nodal dissection. Pathology revealed mucoepidermoid cutaneous carcinoma) comprises a dermal appendage neoplasm (acrospiroma -- of hydra group of rare epidermal, juxta-epidermal, and dermal adenoma type), well-circumscribed, with mitotic figures ductal carcinomas that may coexist with their benign (< 2/hpf). -

Current Diagnosis and Treatment Options for Cutaneous Adnexal Neoplasms with Apocrine and Eccrine Differentiation
International Journal of Molecular Sciences Review Current Diagnosis and Treatment Options for Cutaneous Adnexal Neoplasms with Apocrine and Eccrine Differentiation Iga Płachta 1,2,† , Marcin Kleibert 1,2,† , Anna M. Czarnecka 1,* , Mateusz Spałek 1 , Anna Szumera-Cie´ckiewicz 3,4 and Piotr Rutkowski 1 1 Department of Soft Tissue/Bone Sarcoma and Melanoma, Maria Sklodowska-Curie National Research Institute of Oncology, 02-781 Warsaw, Poland; [email protected] (I.P.); [email protected] (M.K.); [email protected] (M.S.); [email protected] (P.R.) 2 Faculty of Medicine, Medical University of Warsaw, 02-091 Warsaw, Poland 3 Department of Pathology and Laboratory Diagnostics, Maria Sklodowska-Curie National Research Institute of Oncology, 02-781 Warsaw, Poland; [email protected] 4 Department of Diagnostic Hematology, Institute of Hematology and Transfusion Medicine, 00-791 Warsaw, Poland * Correspondence: [email protected] or [email protected] † Equally contributed to the work. Abstract: Adnexal tumors of the skin are a rare group of benign and malignant neoplasms that exhibit morphological differentiation toward one or more of the adnexal epithelium types present in normal skin. Tumors deriving from apocrine or eccrine glands are highly heterogeneous and represent various histological entities. Macroscopic and dermatoscopic features of these tumors are unspecific; therefore, a specialized pathological examination is required to correctly diagnose patients. Limited Citation: Płachta, I.; Kleibert, M.; treatment guidelines of adnexal tumor cases are available; thus, therapy is still challenging. Patients Czarnecka, A.M.; Spałek, M.; should be referred to high-volume skin cancer centers to receive an appropriate multidisciplinary Szumera-Cie´ckiewicz,A.; Rutkowski, treatment, affecting their outcome. -
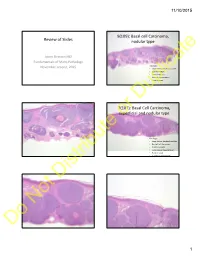
Basal Cell Carcinoma, Superficial and Nodular Typeor
11/10/2015 9(109): Basal cell Carcinoma, Review of Slides nodular type Jason Stratton MD Fundamentals of Mohs Pathology Histology: November second, 2015 • Large dermal basaloid nodules • Cleft formation • Central necrosis • Myxoid degeneration • Focal dropout Duplicate 7(107): Basal Cell Carcinoma, superficial and nodular typeor Histology: • Large dermal basaloid nodules • No cleft left formation • Central necrosis • Focal myxoid degeneration • Focal dropout • Superficial component Distribute Not Do 1 11/10/2015 4(104): BCC Nodular and Infiltrative Histology: Clinically: • Large dermal basaloid nodules • Upper back and face most • Cleft formation common • No central necrosis • Poorly defined plaque • Prominent myxoid degeneration • Infiltrative nests without dense Perineural invasion is more fibrosis common Duplicate 6(106): BCC Nodular and Follicular or Histology: • Variable sized dermal nodules • Focal cleft formation • Contain an arborizing pattern without the dispersion or fibrosis of micronodular 2(101): BCC NodulocysticDistribute Not Histology: • Large sized dermal nodules with central myxoid degeneration or necrosis. As a term Nodulocystic includes both Do nodular and Cystic varieties 2 11/10/2015 5(105): BCC Nodulocystic Histology: • Variably sized dermal nodules • Myxoid degeneration with mucin accumulation • Alcian Blue positive at pH 2.5 Duplicate 3(102): BCC, Fibroepitheliomatous type or Histology: • Arborizing networks and cords of basaloid • Variably basaloid with cleft formation Distribute 1(100): BCC, Matrical type Not -

A 5 Year Histopathological Study of Skin Adnexal Tumors at a Tertiary Care Hospital
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 14, Issue 4 Ver. VII (Apr. 2015), PP 01-05 www.iosrjournals.org A 5 Year Histopathological Study of Skin Adnexal Tumors at a Tertiary Care Hospital Dr.Vani.D1, Dr.Ashwini.N.S2, Dr.Sandhya.M3, Dr.T.R.Dayananda4, Dr.Bharathi.M5 1,2,3,5, Department of Pathology, Mysore Medical College & Research Institute, Mysore, India 4, Department of Dermatology, BGS Apollo Hospital, Mysore, India Abstract: Introduction: Skin adnexal neoplasms are uncommon and are daunting diagnostic problems in view of the wide spectrum of lesions and their variants. Benign adnexal neoplasms are more common than malignant lesions. Aim: To study histopathology of skin adnexal neoplasms and to correlate with the clinical profile. Methodology: 51cases with a diagnosis of skin adnexal neoplasm over a 5 year period reported in the Department of Pathology, Mysore Medical College & Research Institute were included in the study. Histopathological examination was done on Haematoxylin& Eosin stained slides and corroborated with special stains wherever required. Results: Skin adnexal tumors were most common in the age group of 40 to 49 years (21.56%, 11/51). Male to female ratio was 1:1.68. The head and neck region was the most common site affected (64.70%, 33/51) with 39.21% (20/51) caseslocated on the face. 74.50% (38/51) cases were benign and 25.49% (13/51) cases were malignant. The sweat gland tumors formed the largest group involving 43.13% (22/51) cases followed by the hair follicle tumors 37.25% (19/51) followed by sebaceous gland tumors 19.60% (10/51). -

Tumours of the Pilosebaceous Unit
Downloaded from jcp.bmj.com on 29 February 2008 Skin adnexal neoplasms—part 1: An approach to tumours of the pilosebaceous unit K O Alsaad, N A Obaidat and D Ghazarian J. Clin. Pathol. 2007;60;129-144; originally published online 1 Aug 2006; doi:10.1136/jcp.2006.040337 Updated information and services can be found at: http://jcp.bmj.com/cgi/content/full/60/2/129 These include: References This article cites 75 articles, 4 of which can be accessed free at: http://jcp.bmj.com/cgi/content/full/60/2/129#BIBL Rapid responses You can respond to this article at: http://jcp.bmj.com/cgi/eletter-submit/60/2/129 Email alerting Receive free email alerts when new articles cite this article - sign up in the box at the service top right corner of the article Notes To order reprints of this article go to: http://journals.bmj.com/cgi/reprintform To subscribe to Journal of Clinical Pathology go to: http://journals.bmj.com/subscriptions/ Downloaded from jcp.bmj.com on 29 February 2008 129 REVIEW Skin adnexal neoplasms—part 1: An approach to tumours of the pilosebaceous unit K O Alsaad, N A Obaidat, D Ghazarian ................................................................................................................................... See linked review p145 J Clin Pathol 2007;60:129–144. doi: 10.1136/jcp.2006.040337 Skin adnexal neoplasms comprise a wide spectrum of benign NORMAL HISTOLOGY OF SKIN and malignant tumours that exhibit morphological APPENDAGES Skin appendages are derived from the ectoderm, differentiation towards one or more types of adnexal structures and start to develop early during the embryological found in normal skin. -

Sebaceous Differentiation in Poroid Neoplasms: Report of 11 Cases, Including a Case of Metaplastic Carcinoma Associated with Apocrine Poroma (Sarcomatoid Apocrine Porocarcinoma)
Kazakov, D V; Kutzner, H; Spagnolo, D V; Kempf, W; Zelger, B; Mukensnabl, P; Michal, M (2008). Sebaceous differentiation in poroid neoplasms: report of 11 cases, including a case of metaplastic carcinoma associated with apocrine poroma (sarcomatoid apocrine porocarcinoma). American Journal of Dermatopathology, 30(1):21-26. Postprint available at: http://www.zora.uzh.ch University of Zurich Posted at the Zurich Open Repository and Archive, University of Zurich. Zurich Open Repository and Archive http://www.zora.uzh.ch Originally published at: American Journal of Dermatopathology 2008, 30(1):21-26. Winterthurerstr. 190 CH-8057 Zurich http://www.zora.uzh.ch Year: 2008 Sebaceous differentiation in poroid neoplasms: report of 11 cases, including a case of metaplastic carcinoma associated with apocrine poroma (sarcomatoid apocrine porocarcinoma) Kazakov, D V; Kutzner, H; Spagnolo, D V; Kempf, W; Zelger, B; Mukensnabl, P; Michal, M Kazakov, D V; Kutzner, H; Spagnolo, D V; Kempf, W; Zelger, B; Mukensnabl, P; Michal, M (2008). Sebaceous differentiation in poroid neoplasms: report of 11 cases, including a case of metaplastic carcinoma associated with apocrine poroma (sarcomatoid apocrine porocarcinoma). American Journal of Dermatopathology, 30(1):21-26. Postprint available at: http://www.zora.uzh.ch Posted at the Zurich Open Repository and Archive, University of Zurich. http://www.zora.uzh.ch Originally published at: American Journal of Dermatopathology 2008, 30(1):21-26. Sebaceous differentiation in poroid neoplasms: report of 11 cases, including a case of metaplastic carcinoma associated with apocrine poroma (sarcomatoid apocrine porocarcinoma) Abstract We describe 11 poroid neoplasms with sebaceous differentiation, including a metaplastic (sarcomatoid) carcinoma arising in association with an apocrine poroma. -

Angiosarcomas
Angiosarcomas recurrence after surgical excision and radiother- Elisa Cinotti, Franco Rongioletti apy. In one case, the accompanying dense infl am- matory infi ltrate was attributable to a superimposed Cutaneous angiosarcoma is a rare, aggressive infection by Pseudomonas aeruginosa . vascular sarcoma that occurs in three main differ- Pathology : It is characterized by the same ent clinical settings: classic cutaneous angiosar- atypical vessels of the classical angiosarcoma, coma arising in sun-damaged skin of elderly with the addition of a prominent infl ammatory patients, cutaneous angiosarcoma associated lymphoid infi ltrate between the vessels, obliterat- with chronic lymphedema, and post radiation ing some or most of the channels (Fig. 2 ). The angiosarcoma. Recent studies have shown that infi ltrate can be diffuse or can be organized in high-level amplifi cation of MYC oncogene seems lymphoid follicles with germinal centers scat- to be specifi c for radiation and lymphedema- tered within the diffuse lymphoid infi ltrate. associated angiosarcoma. A new histological Vessels are poorly circumscribed, irregularly variant has been named pseudolymphomatous dilated, and anastomosing, lined by prominent, cutaneous angiosarcoma. In general, cutaneous atypical endothelial cells (Fig. 3 , 4 ) that usually angiosarcoma carries a poor prognosis, associ- express CD31 (Fig. 5 ), CD34, and D2-40. Most ated with 5-year overall survival rates between 10 of the cells of the lymphoid infi ltrate express and 30 %. strong immunoreactivity for CD3, CD4, CD5, Pseudolymphomatous angiosarcomas and CD45 markers, whereas only scattered cells Synonyms: Angiosarcoma with prominent express CD8. Most of the lymphocytes of the lymphocytic infi ltrate. germinal centers are positive for CD20, CD21, Introduction: Pseudolymphomatous cutane- CD79a, and Bcl-6 whereas Bcl-2 can be detected ous angiosarcoma, described by Requena et al .