Apparent Survival of Two Trans-Equatorial Migrant Seabirds Breeding in Canada
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beaufort Seas West To
71° 162° 160° 158° 72° U LEGEND 12N 42W Ch u $ North Slope Planning Area ckchi Sea Conservation System Unit (Offset for display) Pingasagruk (abandoned) WAINWRIGHT Atanik (Abandoned) Naval Arctic Research Laboratory USGS 250k Quad Boundaries U Point Barrow I c U Point Belcher 24N Township Boundaries y 72° Akeonik (Ruins) Icy Cape U 17W C 12N Browerville a Trans-Alaska Pipeline p 39W 22N Solivik Island e Akvat !. P Ikpilgok 20W Barrow Secondary Roads (unpaved) a Asiniak!. Point s MEADE RIVER s !. !. Plover Point !. Wainwright Point Franklin !. Brant Point !. Will Rogers and Wiley Post Memorial Whales1 U Point Collie Tolageak (Abandoned) 9N Point Marsh Emaiksoun Lake Kilmantavi (Abandoned) !. Kugrua BayEluksingiak Point Seahorse Islands Bowhead Whale, Major Adult Area (June-September) 42W Kasegaluk Lagoon West Twin TekegakrokLake Point ak Pass Sigeakruk Point uitk A Mitliktavik (Abandoned) Peard Bay l U Ikroavik Lake E Tapkaluk Islands k Wainwright Inlet o P U Bowhead Whale, Major Adult Area (May) l 12N k U i i e a n U re Avak Inlet Avak Point k 36W g C 16N 22N a o Karmuk Point Tutolivik n U Elson Lagoon t r !. u a White (Beluga) Whale, Major Adult Area (September) !. a !. 14N m 29W 17W t r 17N u W Nivat Point o g P 32W Av k a a Nokotlek Point !. 26W Nulavik l A s P v a a s Nalimiut Point k a k White (Beluga) Whale, Major Adult Area (May-September) MEADE RIVER p R s Pingorarok Hill BARROW U a Scott Point i s r Akunik Pass Kugachiak Creek v ve e i !. -

Grand Manan Channel – Southern Part NOAA Chart 13392
BookletChart™ Grand Manan Channel – Southern Part NOAA Chart 13392 A reduced-scale NOAA nautical chart for small boaters When possible, use the full-size NOAA chart for navigation. Published by the 33-foot unmarked rocky patch known as Flowers Rock, 3.9 miles west- northwestward of Machias Seal Island, the channel is free and has a National Oceanic and Atmospheric Administration good depth of water. The tidal current velocity is about 2.5 knots and National Ocean Service follows the general direction of the channel. Daily predictions are given Office of Coast Survey in the Tidal Current Tables under Bay of Fundy Entrance. Off West Quoddy Head, the currents set in and out of Quoddy Narrows, forming www.NauticalCharts.NOAA.gov strong rips. Sailing vessels should not approach West Quoddy Head too 888-990-NOAA closely with a light wind. North Atlantic Right Whales.–The Bay of Fundy is a feeding and nursery What are Nautical Charts? area for endangered North Atlantic right whales (peak season: July through October) and includes the Grand Manan Basin, a whale Nautical charts are a fundamental tool of marine navigation. They show conservation area designated by the Government of Canada. (See North water depths, obstructions, buoys, other aids to navigation, and much Atlantic Right Whales, chapter 3, for more information on right whales more. The information is shown in a way that promotes safe and and recommended measures to avoid collisions with whales.) efficient navigation. Chart carriage is mandatory on the commercial Southwest Head, the southern extremity of Grand Manan Island, is a ships that carry America’s commerce. -

Real Property Issues in the Marine Aquaculture Industry in New Brunswick
REAL PROPERTY ISSUES IN THE MARINE AQUACULTURE INDUSTRY IN NEW BRUNSWICK SUE NICHOLS IAN EDWARDS JIM DOBBIN KATALIN KOMJATHY SUE HANHAM October 2001 TECHNICAL REPORT NO. 208217 REAL PROPERTY ISSUES IN THE MARINE AQUACULTURE INDUSTRY IN NEW BRUNSWICK Sue Nichols Ian Edwards Jim Dobbin Katalin Komjathy Sue Hanham Department of Geodesy and Geomatics Engineering University ofNew Brunswick P.O. Box 4400 Fredericton, N.B. Canada E3B 5A3 October 2001 PREFACE In order to make our extensive series of technical reports more readily available, we have scanned the old master copies and produced electronic versions in Portable Document Format. The quality of the images varies depending on the quality of the originals. The images have not been converted to searchable text. PREFACE This report was prepared under contract for the New Brunswick Department of Fisheries and Aquaculture. The research was carried out in 1997 at the University of New Brunswick, Fredericton, Canada, under the leadership of Professor Sue Nichols. As with any copyrighted material, permission to reprint or quote extensively from this report must be received from the authors. The citation to this work should appear as follows: Nichols, S., I. Edwards, J. Dobbin, K. Komjathy, and S. Hanham (2001). Real Property Issues in the Marine Aquaculture Industry in New Brunswick. Final contract report for the New Brunswick Department of Fisheries and Aquaculture, by the Geographical Engineering Laboratory, Department of Geodesy and Geomatics Engineering Technical Report No. 208, University of New Brunswick, Fredericton, New Brunswick, Canada, 102 pp. Real Property Issues in the Marine Aquaculture Industry in New Brunswick Acknowledgements The authors would like to thank for the Department of Fisheries and Aquaculture for recognising the need for this research and providing the funds. -

International Black-Legged Kittiwake Conservation Strategy and Action Plan Acknowledgements Table of Contents
ARCTIC COUNCIL Circumpolar Seabird Expert Group July 2020 International Black-legged Kittiwake Conservation Strategy and Action Plan Acknowledgements Table of Contents Executive Summary ..............................................................................................................................................4 CAFF Designated Agencies: Chapter 1: Introduction .......................................................................................................................................5 • Norwegian Environment Agency, Trondheim, Norway Chapter 2: Ecology of the kittiwake ....................................................................................................................6 • Environment Canada, Ottawa, Canada Species information ...............................................................................................................................................................................................6 • Faroese Museum of Natural History, Tórshavn, Faroe Islands (Kingdom of Denmark) Habitat requirements ............................................................................................................................................................................................6 • Finnish Ministry of the Environment, Helsinki, Finland Life cycle and reproduction ................................................................................................................................................................................7 • Icelandic Institute of Natural -
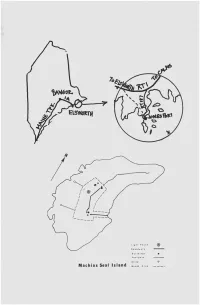
Machias Seal Island Mowad a R I MACHIAS SEAL ISLABD
Foet patK Bltn4 Machias Seal Island Mowad A r i MACHIAS SEAL ISLABD by Paula Butler, Belmont Machias Seal Island— the Contested Island*— has served as a sentinel at the South end of the Bay of Fundy slnce the estahlishment of a lighthouse in 1832. Before that time, this rock (barren except for a highland meadow in summer) vas a marinar's nightmare of sudden fog banks, high seas and hidden rocky shoals. The Harbour Seal, for whioh this island vas probably named, occurs only at a nearby shoal, North Rock. Although today's Journey is quite safe, trips to the island are cancelled vithout notice due to unexpected vind changes or a possible dangerous landing because of ground svells, strong cur- rents and surf. The meadov area of the island contains a variety of plant life: asters, vild parsleys, docks, grasses, sedges, and many other herbs. This island, like many others in the Machias Bay area, vas used for cattle and sheep grazing as vell as Limited farming. Some of these islands vere strategic in the naval maneuvers during the American Revolution. The first naval encounter of that var vas in Machias Bay vhen the villagers of Machias beached the British cutter Margareta. Most birders and photographers are attracted to Machias Seal Island to ob serve Common Puffins (Pratercula árctica) at cióse range. Common Puffins and Razorbills (Alca torda) seem to invite us not only to vatch them but to take delight in posing. Tvo blinds eire provided overlooking the rocky nesting area. The puffins arrive in late April and remain in the vater until they receive a mysterious signal; then, in one large flock, they settle into their nesting sites. -

Strategic Environmental Assessment for the Orphan Basin
Orphan Basin Strategic Environmental Assessment Prepared by Prepared for Canada-Newfoundland Offshore Petroleum Board Fifth Floor, TD Place 140 Water Street St. John’s, NL A1C 6H6 11 November 2003 SA767 Orphan Basin Strategic Environmental Assessment Prepared by Prepared for Canada-Newfoundland Offshore Petroleum Board Fifth Floor, TD Place 140 Water Street St. John’s, NL A1C 6H6 11 November 2003 SA767 Orphan Basin Strategic Environmental Assessment Prepared by LGL Limited environmental research associates 388 Kenmount Road St. John’s, NL A1B 4A5 709 754-1992 (p) 709 754-7718 (f) Prepared for Canada-Newfoundland Offshore Petroleum Board Fifth Floor, TD Place 140 Water Street St. John’s, NL A1C 6H6 11 November 2003 SA767 Table of Contents Page Table of Contents........................................................................................................................................ ii List of Tables ............................................................................................................................................. xi List of Figures........................................................................................................................................... xii 1.0 Introduction..................................................................................................................................... 1 2.0 Oil and Gas Activities in Orphan Basin.......................................................................................... 3 2.1 Rights Issuance Process and Call for Bids......................................................................... -

Biodiversity of Michigan's Great Lakes Islands
FILE COPY DO NOT REMOVE Biodiversity of Michigan’s Great Lakes Islands Knowledge, Threats and Protection Judith D. Soule Conservation Research Biologist April 5, 1993 Report for: Land and Water Management Division (CZM Contract 14C-309-3) Prepared by: Michigan Natural Features Inventory Stevens T. Mason Building P.O. Box 30028 Lansing, MI 48909 (517) 3734552 1993-10 F A report of the Michigan Department of Natural Resources pursuant to National Oceanic and Atmospheric Administration Award No. 309-3 BIODWERSITY OF MICHIGAN’S GREAT LAKES ISLANDS Knowledge, Threats and Protection by Judith D. Soule Conservation Research Biologist Prepared by Michigan Natural Features Inventory Fifth floor, Mason Building P.O. Box 30023 Lansing, Michigan 48909 April 5, 1993 for Michigan Department of Natural Resources Land and Water Management Division Coastal Zone Management Program Contract # 14C-309-3 CL] = CD C] t2 CL] C] CL] CD = C = CZJ C] C] C] C] C] C] .TABLE Of CONThNTS TABLE OF CONTENTS I EXECUTIVE SUMMARY iii INTRODUCTION 1 HISTORY AND PHYSICAL RESOURCES 4 Geology and post-glacial history 4 Size, isolation, and climate 6 Human history 7 BIODWERSITY OF THE ISLANDS 8 Rare animals 8 Waterfowl values 8 Other birds and fish 9 Unique plants 10 Shoreline natural communities 10 Threatened, endangered, and exemplary natural features 10 OVERVIEW OF RESEARCH ON MICHIGAN’S GREAT LAKES ISLANDS 13 Island research values 13 Examples of biological research on islands 13 Moose 13 Wolves 14 Deer 14 Colonial nesting waterbirds 14 Island biogeography studies 15 Predator-prey -

2 Study Area
CA PDF Page 49 of 1038 Energy East Project Part B: Deterministic Modelling of the Ecological and Volume 24: Ecological and Human Health Risk Human Health Consequences of Marine Oil Spills Assessment for Oil Spills in the Marine Environment Section 2: Study Area 2 STUDY AREA The study area for the analysis of hypothetical crude oil spills for the proposed marine terminal is described in detail in Part A, Section 2 of the EHHRA. This section provides an overview. 2.1 Overview of Canaport Energy East Marine Terminal Operations and Expanded Shipping Operations The proposed marine terminal will be located on the western shore of the Bay of Fundy, southeast of the City of Saint John and southwest of Mispec Point, New Brunswick. The marine terminal is proposed to be located adjacent to the two existing Canaport marine facilities: a single buoy mooring (SBM or mono- buoy) for offloading crude oil to the Irving Canaport facility, and the Canaport LNG terminal for import and now recently licensed for export of liquefied natural gas (Figure 1-1). The marine terminal will include pile-supported trestles and breasting/mooring dolphins for two berths that can accommodate Aframax and Suezmax tankers (Berth 2), as well as VLCC tanker types (Berth 1), with capacities of 113,300 to 348,000 m3 (710,000 to 2.2 million barrels). The two berths will be constructed simultaneously. Oil will be pumped from shore via a trestle approximately 645 m long. Berths 1 and 2 will be interconnected by a trestle approximately 380 m long. The marine terminal is expected to receive approximately 281 tanker calls per year for shipping of crude oil products originating from western Canada. -

Polar Continental Shelf Program Science Report 2019: Logistical Support for Leading-Edge Scientific Research in Canada and Its Arctic
Polar Continental Shelf Program SCIENCE REPORT 2019 LOGISTICAL SUPPORT FOR LEADING-EDGE SCIENTIFIC RESEARCH IN CANADA AND ITS ARCTIC Polar Continental Shelf Program SCIENCE REPORT 2019 Logistical support for leading-edge scientific research in Canada and its Arctic Polar Continental Shelf Program Science Report 2019: Logistical support for leading-edge scientific research in Canada and its Arctic Contact information Polar Continental Shelf Program Natural Resources Canada 2464 Sheffield Road Ottawa ON K1B 4E5 Canada Tel.: 613-998-8145 Email: [email protected] Website: pcsp.nrcan.gc.ca Cover photographs: (Top) Ready to start fieldwork on Ward Hunt Island in Quttinirpaaq National Park, Nunavut (Bottom) Heading back to camp after a day of sampling in the Qarlikturvik Valley on Bylot Island, Nunavut Photograph contributors (alphabetically) Dan Anthon, Royal Roads University: page 8 (bottom) Lisa Hodgetts, University of Western Ontario: pages 34 (bottom) and 62 Justine E. Benjamin: pages 28 and 29 Scott Lamoureux, Queen’s University: page 17 Joël Bêty, Université du Québec à Rimouski: page 18 (top and bottom) Janice Lang, DRDC/DND: pages 40 and 41 (top and bottom) Maya Bhatia, University of Alberta: pages 14, 49 and 60 Jason Lau, University of Western Ontario: page 34 (top) Canadian Forces Combat Camera, Department of National Defence: page 13 Cyrielle Laurent, Yukon Research Centre: page 48 Hsin Cynthia Chiang, McGill University: pages 2, 8 (background), 9 (top Tanya Lemieux, Natural Resources Canada: page 9 (bottom -

Seeing the Light: Report on Staffed Lighthouses in Newfoundland and Labrador and British Columbia
SEEING THE LIGHT: REPORT ON STAFFED LIGHTHOUSES IN NEWFOUNDLAND AND LABRADOR AND BRITISH COLUMBIA Report of the Standing Senate Committee on Fisheries and Oceans The Honourable Fabian Manning, Chair The Honourable Elizabeth Hubley, Deputy Chair October 2011 (first published in December 2010) For more information please contact us by email: [email protected] by phone: (613) 990-0088 toll-free: 1 800 267-7362 by mail: Senate Committee on Fisheries and Oceans The Senate of Canada, Ottawa, Ontario, Canada, K1A 0A4 This report can be downloaded at: http://senate-senat.ca Ce rapport est également disponible en français. MEMBERSHIP The Honourable Fabian Manning, Chair The Honourable Elizabeth Hubley, Deputy Chair and The Honourable Senators: Ethel M. Cochrane Dennis Glen Patterson Rose-Marie Losier-Cool Rose-May Poirier Sandra M. Lovelace Nicholas Vivienne Poy Michael L. MacDonald Nancy Greene Raine Donald H. Oliver Charlie Watt Ex-officio members of the committee: The Honourable Senators James Cowan (or Claudette Tardif) Marjory LeBreton, P.C. (or Claude Carignan) Other Senators who have participated on this study: The Honourable Senators Andreychuk, Chaput, Dallaire, Downe, Marshall, Martin, Murray, P.C., Rompkey, P.C., Runciman, Nancy Ruth, Stewart Olsen and Zimmer. Parliamentary Information and Research Service, Library of Parliament: Claude Emery, Analyst Senate Committees Directorate: Danielle Labonté, Committee Clerk Louise Archambeault, Administrative Assistant ORDER OF REFERENCE Extract from the Journals of the Senate, Sunday, June -

Triassic Basin Stratigraphy at Grand Manan, New Brunswick, Canada J
Document generated on 09/27/2021 4:33 p.m. Atlantic Geology Triassic Basin Stratigraphy at Grand Manan, New Brunswick, Canada J. Gregory. McHone Volume 47, 2011 Article abstract The island of Grand Manan (Canada) in the southwestern Bay of Fundy has the URI: https://id.erudit.org/iderudit/ageo47art06 only exposed strata and basalt of the Grand Manan Basin, a mainly submerged Early Mesozoic riſt basin about 30 km wide by 70 km long. The basin is See table of contents bounded on the southeast by the west-dipping Red Point Fault, which bisects the island, and on the northwest by a submarine border fault marked by the Murr Escarpment, a bathymetric feature that parallels the coast of Maine Publisher(s) (USA). A fault-bounded horst of Ediacaran to Cambrian rocks separates the Grand Manan Basin from the much larger Fundy Basin to the east. The Atlantic Geoscience Society Ashburton Head, Seven Days Work, and Southwest Head members of the end-Triassic Dark Harbour Basalt cover most of western Grand Manan with a ISSN total thickness around 240 m. Up to 12 m of sub-horizontal grey mudstone and fine-grained red sandstone of the Dwellys Cove Formation are exposed along 0843-5561 (print) the western shoreline beneath the basalt. Coarse red arkosic sandstone a few 1718-7885 (digital) metres thick at Miller Pond Road rests on a basement of Late Ediacaran rocks east of the basin. Exposures of the Dwellys Cove and Miller Pond Road Explore this journal formations are at the top and bottom, respectively, of several km (?) of sub-horizontal Late Triassic clastic basin strata, juxtaposed by the eastern border fault. -

Consolidation of Wildlife Management Units
WILDLIFE ACT LOI SUR LA FAUNE CONSOLIDATION OF WILDLIFE CODIFICATION ADMINISTRATIVE MANAGEMENT UNITS DU RÈGLEMENT SUR LES REGULATIONS SECTEURS DE GESTION DE LA R.R.N.W.T. 1990,c.W-15 FAUNE R.R.T.N.-O. 1990, ch. W-15 AS AMENDED BY MODIFIÉ PAR R-091-93 R-091-93 R-020-96 R-020-96 R-104-98 (CIF 98/08/01) R-104-98 (EEV1998-08-01) This consolidation is not an official statement of the La presénte codification administrative ne constitue law. It is an office consolidation prepared for pas le texte officiel de la loi; elle n’est établie qu'à convenience of reference only. The authoritative text titre documentaire. Seuls les règlements contenus of regulations can be ascertained from the Revised dans les Règlements révisés des Territoires du Nord- Regulations of the Northwest Territories, 1990 and Ouest (1990) et dans les parutions mensuelles de la the monthly publication of Part II of the Northwest Partie II de la Gazette des Territoires du Nord-Ouest Territories Gazette (for regulations made before (dans le cas des règlements pris avant le 1 er avril April 1, 1999) and Part II of the Nunavut Gazette (for 1999) et de la Partie II de la Gazette du Nunavut regulations made on or after April 1, 1999). (dans le cas des règlements pris depuis le 1 er avril 1999) ont force de loi. WILDLIFE ACT LOI SUR LA FAUNE WILDLIFE MANAGEMENT UNITS RÈGLEMENT SUR LES SECTEURS REGULATIONS DE GESTION DE LA FAUNE 1. The wildlife management units shall be delimited 1.