Azobisisobutyronitrile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TOXICOLOGICAL PROFILE for CYANIDE Date Published
0 010024 ATSDR/TP-88/12 TOXICOLOGICAL PROFILE FOR CYANIDE Date Published - December 1989 ' Prepared by: Syracuse Research Corporation under Contract No. 68-CS-0004 for Agency for Toxic Substances and Disease Registry (A TSDR) U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA) Technical editing/document preparation by: Oak Ridge National Laboratory under DOE Interagency Agreement No. 1857-B026-Al DISCLAIMER Mention of company name or product does not constitute endorsement by the Agency for Toxic Substances and Disease Registry. FOREWORD The Superfund Amendments and Reauthorization Act of 1986 (Public Law 99-499) extended and amended the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCI.A or Superfund). This public law (also known as SARA) directed the Agency for Toxic Substances and Disease Registry (ATSDR) to prepare toxicological profiles for hazardous substances which are most commonly found at facilities on the CERCI.A National Priorities List and which pose the most significant potential threat to human health, as determined by ATSDR and the Environmental Protection Agency (EPA). The list of the 100 most significant hazardous substances was published in the Federal Register on Ap r il 17, 1987. Section 110 (3) of SARA directs the Administrator of ATSDR to prepare a toxicological profile for each substance on the list. Each profile must include the following content: "(A) An examination, summary, and interpretation of available toxicological information and epidemiologic evaluations on a hazardous substance in order to ascertain the levels of significant human exposure for the substance and the associated acute, subacute , and chronic health effects. -

(12) United States Patent (10) Patent No.: US 7.449,439 B2 to Et Al
USOO7449439B2 (12) United States Patent (10) Patent No.: US 7.449,439 B2 to et al. (45) Date of Patent: Nov. 11, 2008 (54) WATER-SOLUBLE THICKENER AND LIQUID (56) References Cited ACDIC DETERGENT U.S. PATENT DOCUMENTS (75) Inventors: Kenji Ito, Aichi (JP); Yoshio Mori, 3,768,565 A 10, 1973 Persinski et al. Aichi (JP) (Continued) (73) Assignee: Toagosei Co., Ltd., Tokyo (JP) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this JP 53-463O2 A 4f1978 patent is extended or adjusted under 35 U.S.C. 154(b) by 455 days. (Continued) (21) Appl. No.: 10/530,179 OTHER PUBLICATIONS International Search Report PCT/JP2003/012764 dated Dec. 9, 2003. (22) PCT Filed: Oct. 6, 2003 (Continued) (86). PCT No.: PCT/UP03/12764 Primary Examiner Brian PMruk S371 (c)(1), (74) Attorney, Agent, or Firm Sughrue Mion, PLLC (2), (4) Date: Apr. 4, 2005 (57) ABSTRACT (87) PCT Pub. No.: WO2004/031314 A water-soluble thickener which is highly effective even in PCT Pub. Date: Apr. 15, 2004 thickening strongly acidic aqueous Solutions and has excel lent stability in such solutions. It comprises a water-soluble (65) Prior Publication Data copolymer having a weight-average molecular weight of 6,000,000 or higher obtainable by polymerizing a monomer US 2006/OO46949 A1 Mar. 2, 2006 mixture which comprises 2-acrylamido-2-methylpropane (30) Foreign Application Priority Data Sulfonic acid and/or a salt thereofandacrylic acid and/or a salt thereofas essential components and optionally one or more Oct. 4, 2002 (JP) ............................. 2002-292975 other copolymerizable monomer components including the Oct. -

Hydroxylamines and Hydrazines As Surrogates of Sp3 Carbons in Medicinal Chemistry
Wayne State University Wayne State University Dissertations January 2018 Hydroxylamines And Hydrazines As Surrogates Of Sp3 Carbons In Medicinal Chemistry Sandeep Dhanju Wayne State University, [email protected] Follow this and additional works at: https://digitalcommons.wayne.edu/oa_dissertations Part of the Chemistry Commons Recommended Citation Dhanju, Sandeep, "Hydroxylamines And Hydrazines As Surrogates Of Sp3 Carbons In Medicinal Chemistry" (2018). Wayne State University Dissertations. 2156. https://digitalcommons.wayne.edu/oa_dissertations/2156 This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. HYDROXYLAMINES AND HYDRAZINES AS SURROGATES OF SP3 CARBONS IN MEDICINAL CHEMISTRY by SANDEEP DHANJU DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2019 MAJOR: CHEMISTRY (Organic) Approved By: Advisor Date DEDICATION I dedicate my PhD work to my parents Sanubhai Shrestha and Punamaya Dhanju, for their endless love and support. ii ACKNOWLEDGEMENTS First and foremost, I would like to express my sincere gratitude to my Ph.D. advisor Prof. David Crich for his incessant support and guidance for my Ph.D. research work during the past five years in his laboratory. I am grateful and fortunate to be one of his graduate students. His encouragement and motivation drove me to the end of this thesis, and I was able to develop an in-depth knowledge and enthusiasm in the areas of organic chemistry and medicinal chemistry. I would like to extend my gratitude to Prof. -

Chemical Hygiene Plan Ii Revised 03/2021 Table of Contents
MAR 2021 Office of Environmental Health and Safety Principal Author/Editor: David Webber, PhD/Chemical Hygiene Officer Contributing Authors/Editors: Nikolai Evdokimov, PhD, James Gibson, PhD, Tania Guardado, PhD, Amanda Jevons, Deona Willes, MPH Graphics/Design: Alfred M. Bouziane, MS, Brent Pantell USC Chemical Hygiene Plan ii Revised 03/2021 Table of Contents i.0 2021 Revision Summary Section 3.0 vi Section 4.0 vi Section 5.0 vii Section 7.0 vii Section 8.0 viii Section 10.0 x Appendix D x Appendix G x 1.0 Introduction Purpose and Scope 1.1 Sources of Safety Information 1.2 2.0 Regulatory Requirements 3.0 Roles and Responsibilities Research Safety Oversight Committee (RSOC) 3.1 Campus-Wide Chemical Safety Committee (CCSC) 3.1 Other Safety Committees 3.2 Office of Environmental Health & Safety 3.2 Principal Investigator (PI) 3.3 Training Requirements 3.5 4.0 Basics of Laboratory Safety Hazard, Risk, and Safety Management 4.1 Hierarchy of Safety Controls 4.1 Group Safety Management and Safety Culture 4.4 USC Chemical Hygiene Plan iii Revised 03/2021 Basics of Lab Facilities, Equipment, and Emergency Supplies 4.7 Emergency Equipment and Supplies 4.15 Open Flames 4.20 5.0 Hazard Communication Labeling and Signage Systems 5.2 Labelling and Signage in the Lab: What You Need to Do 5.5 Safety Data Sheets (SDSs): What Are They? 5.7 SDSs in The Lab: What You Need to Do 5.8 6.0 Hazardous Chemicals and Hazard Classification Introduction 6.1 Health-Hazardous Chemicals: Routes of Exposure 6.2 Particularly Hazardous Substances (PHS) 6.16 7.0 Chemical -

Hydrogen Cyanide and Cyanides: Human Health Aspects
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization. Concise International Chemical Assessment Document 61 HYDROGEN CYANIDE AND CYANIDES: HUMAN HEALTH ASPECTS Please note that the layout and pagination of this pdf file are not identical to the version in press First draft prepared by Prof. Fina Petrova Simeonova, Consultant, National Center of Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria; and Dr Lawrence Fishbein, Fairfax, Virginia, USA Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2004 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management -

United States Patent Office 2,918,478 Patented Dec
United States Patent Office 2,918,478 Patented Dec. 22, 1959 2 and non-protonic acids, the vinylene carbonate is poly 2,918,478 merized. The polymerizing agents referred to include: WNYLENE CARBONATE AND METHODS OF Benzoyl peroxide PREPARING T Actyl peroxide Melvin S. Newman, Upper Arlington, Ohio, assignor to Di-t-butyl peroxide corporationThe Ohio Stateof Ohio University Research Foundation, a GN GN Dimethyl azobisisobutyronitrile- CH-i-N-N-(-CH, No Drawing. Original application June 1, 1954, Serial CE CH3 No. 433,795. Divided and this application April 16, O Sulfuric acid 1957, Serial No. 653,053 Hydrochloric acid 6 Claims. (CI. 260-340.2) Hydrofluoric acid Perchloric acid This application is the sole application of Melvin S. Aluminum chloride Newman and is a division of a co-pending joint applica s Zinc chloride tion of Melvin S. Newman and Roger W. Addor, Serial Boron trifluoride No. 433,795, filed June 1, 1954, which itself is in part Hydrogen chloride-aluminum chloride a continuation of copending application Serial No. Hydrogen fluoride-boron trifluoride 338,654, filed February 25, 1953. The invention disclosed in this application relates to 20 On treating with a nitrating agent, an aminating agent, vinylene carbonate; to homo polymers thereof; and to an acetylating agent, an alkylating agent (e.g. an ether derivatives of such vinylene carbonate and of such poly fying agent), a carboxymethylating agent, or with other mers and to processes for the syntheses of such com agents, I react the vinylene carbonate, the polymers, or pounds. the derivatives of such polymers further. One of the objects is therefore the preparation of 25 PREPARATION OF VENYLENE CARBONATE vinylene carbonate. -

Cyanide Hazards to Fish, Wildlife, and Invertebrates: a Synoptic Review
Biological Report 85(1.23) Contaminant Hazard Reviews December 1991 Report 23 Cyanide Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review by Ronald Eisler U.S. Fish and Wildlife Service Patuxent Wildlife Research Center Laurel, Maryland 20708 Biological Report This publication series of the Fish and Wildlife Service comprises reports on the results of research, developments in technology, and ecological surveys and inventories of effects of land-use changes on fishery and wildlife resources. They may include proceedings of workshops, technical conferences, or symposia; and interpretive bibliographies. ISSN 0895-1926 Copies of this publication may be purchased from the National Technical Information Service (NTIS), 5285 Port Royal Road, Springfield, VA 22161. This publication may be cited as: Eisler, Ronald. 1991. Cyanide Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review- U.S. Fish Wildl. Serv., Biol. Rep. 85(1.23). Abstract Chemical Properties Mode of Action Clinical Features Antidotes Sources and Uses Background Concentrations Persistence in Water, Soil, and Air Lethal and Sublethal Effects Terrestrial Flora and Invertebrates Aquatic Organisms Birds Mammals Recommendations Acknowledgments References Tables Number 1 Some properties of potassium cyanide, hydrogen cyanide, and sodium cyanide 2 Background concentrations of cyanide in selected living resources and in nonbiological materials 3 Cyanide effects on selected species of aquatic organisms 4 Cyanide effects on selected species of birds 5 Cyanide effects on selected species of mammals 6 Proposed free cyanide criteria for the protection of living resources and human health 2 Cyanide Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review by Ronald Eisler U.S. Fish and Wildlife Service Patuxent Wildlife Research Center Laurel, Maryland 20708 Abstract. -

Polymer Synthesis and Characterization
Basic Laboratory Course For Polymer Science M. Sc. Program POLYMER SYNTHESIS AND CHARACTERIZATION WS 2011/12 Department of Chemistry and Biochemistry Free University of Berlin Edited by Florian Paulus, Dirk Steinhilber, Tobias Becherer Polymer Synthesis and Characterization – Basic Laboratory Course Table of Contents 1. Experiments .......................................................................................................... 1 1.1. Free Radical Chain Growth Polymerization .......................................................... 1 1.1.1. Bulk Polymerization of MMA with AIBN (Trommsdorff-Norrish Effect) ............... 1 1.1.2. Suspension Polymerization of Styrene with DBPO ............................................... 3 1.1.3. Emulsion Polymerization of Styrene ..................................................................... 4 1.1.4. Polymer Analogues Reaction: Synthesis and Characterization of Sodium Carboxymethylcellulose ........................................................................................ 5 1.1.5. Free Radical Copolymerization of MMA and Styrene ........................................... 6 1.1.6. Precipitation Polymerization of Acrylonitrile with Redox Initiator in Water........ 7 1.1.7. Photo polymerization of PEG-400-diacrylate ....................................................... 9 1.1.8. Dilatometry ......................................................................................................... 10 1.2. Anionic Polymerization ...................................................................................... -

Direct Cyanation, Hydrocyanation, Dicyanation And
RSC Advances REVIEW View Article Online View Journal | View Issue Direct cyanation, hydrocyanation, dicyanation and cyanofunctionalization of alkynes Cite this: RSC Adv., 2020, 10,10232 Lifen Peng, *a Zhifang Hu,a Hong Wang,a Li Wu,a Yinchun Jiao,a Zilong Tanga and Xinhua Xu *b In this review, direct cyanation, hydrocyanation, dicyanation, cyanofunctionalization and other cyanation reactions of alkynes were highlighted. Firstly, the use of nitriles and development of cyanation was simply introduced. After presenting the natural properties of alkynes, cyanation reactions of alkynes were classified and introduced in detail. Transition metal catalysed direct cyanation and hydrocyanation of Received 10th February 2020 alkynes gave alkynyl cyanides and alkenyl nitriles in good yields. Dicyanation of alkynes produced 1,2- Accepted 4th March 2020 dicyano adducts. Cyanofunctionalization of alkynes afforded functional cyanated compounds. DOI: 10.1039/d0ra01286f Thiocyanation and selenocyanation yielded the expected functional vinylthiocyanates and rsc.li/rsc-advances vinylselenocyanates. A plausible reaction mechanism is presented if available. Creative Commons Attribution-NonCommercial 3.0 Unported Licence. Introduction benzyl cyanide,10 acetonitrile,11 N,N-dimethylformamide (DMF),12 azobisisobutyronitrile (AIBN),13 trimethylsilylcyanide (TMSCN),14 15 The nitrile group, an equivalent to carbonyl, carboxyl, amino and ary(cyano)iodonium triates, N-cyano-N-phenyl-p-toluenesulfo- 16 17 hydroxymethyl groups, has been considered as not only a key namide -

Copolymer Comprising Units of Type a Deriving from Carboxylic Acid Monomers and Units of Type B Deriving from Sulfonic Acid Monomers
(19) TZZ ¥_T (11) EP 2 896 637 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 22.07.2015 Bulletin 2015/30 C08F 293/00 (2006.01) C11D 3/37 (2006.01) (21) Application number: 14305084.7 (22) Date of filing: 21.01.2014 (84) Designated Contracting States: • Wilson, David James AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 60580 Coye la Foret (FR) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • Clavier, Julie PL PT RO RS SE SI SK SM TR 92600 Asnieres sur Seine (FR) Designated Extension States: • van Gramberen, Eric BA ME 95870 Bezons (FR) (71) Applicant: Rhodia Operations (74) Representative: Nony 93306 Aubervilliers Cedex (FR) 3, rue de Penthièvre 75008 Paris (FR) (72) Inventors: •Orizet,Céline 92340 Bourg la Reine (FR) (54) Copolymer comprising units of type A deriving from carboxylic acid monomers and units of type B deriving from sulfonic acid monomers (57) The present invention relates to a copolymer of type A and B with a molar ratio of units of type A to comprising units of type A deriving from carboxylic acid units of type B greater or equal to 1, wherein said units monomers and units of type B deriving from sulfonic acid of type A and units of type B are statistically distributed, monomers, said units of type A and B representing more said second block having a degree of polymerization DP 2 than 80 mol % of the total moles of units in the copolymer, such that DP2/DP1 ≥ 1. -
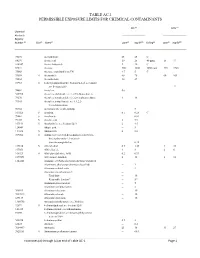
Table Ac-1 Permissible Exposure Limits for Chemical Contaminants
TABLE AC-1 PERMISSIBLE EXPOSURE LIMITS FOR CHEMICAL CONTAMINANTS PEL (d) STEL (o) Chemical Abstracts Registry Number (a) (b) (c) (e) 3(f) (g) (e) 3(f) Skin Name ppm mg/M Ceiling ppm mg/M 75070 Acetaldehyde 25 45 C 64197 Acetic acid 10 25 40 ppm 15 37 108247 Acetic Anhydride 5 20 C 67641 Acetone 500 1200 3000 ppm 750 1780 75868 Acetone cyanohydrin as CN 4.7 5 C 75058 S Acetonitrile 40 70 60 105 98862 Acetophenone 10 49 53963 S 2-Acetylaminofluorene; N-fluoren-2-yl acetamide; see Section 5209 12 74862 Acetylene (h) 540590 Acetylene dichloride; see 1,2-Dichloroethylene 79276 Acetylene tetrabromide:1,1,2,2-tetrabromoethane 1 14 79345 Acetylene tetrachloride; see 1,1,2,2- Tetrachloroethane 50782 Acetylsalicylic acid (Aspirin) 5 107028 S Acrolein 0.1 0.25 C 79061 S Acrylamide -- 0.03 79107 S Acrylic acid 2 5.9 107131 S Acrylonitrile; see Section 5213 2 4.5 124049 Adipic acid -- 5 111693 S Adiponitrile 2 8.8 309002 S Aldrin; 1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a- hexahydro-endo-1,2-exo-5,8- dimethanonaphthalene -- 0.25 107186 S Allyl alcohol 0.5 1.25 4 10 107051 Allyl chloride 1 3 2 6 106923 S Allyl glycidyl ether; AGE 0.2 0.93 2179591 Allyl propyl disulfide 2 12 3 18 1344281 Alumina; see Particulates not otherwise regulated Aluminum, alkyls (not otherwise classified) -- 2 Aluminum soluble salts -- 2 Aluminum metal and oxide -- Total dust -- 10 Respirable fraction(n) -- 5(n) Aluminum pyro powders -- 5 Aluminum welding fumes -- 5 300925 Aluminum distearate -- 10 7047849 Aluminum stearate -- 10 637127 Aluminum tristearate -- 10 1300738 Aminodimethylbenzene; -

UNITED STATES PATENT of FICE PREPARATION of Succinonitrile Charles E
Patented Apr. 3, 1951 2547,686 UNITED STATES PATENT of FICE PREPARATION oF sUCCINoNITRILE Charles E. Brockway, Akron, Ohio, assignor to The B. F. Goodrich Company, New York, N.Y., a. corporation of New York No Drawing. Application December 2, 1949, Serial No. 130,866 5 Claims. (CI. 260-465.8) 2 This invention relates to the preparation of -a- line earth metal hydroxides such as sodium, succinonitrile and more specifically pertains to potassium and calcium hydroxides and salts of the preparation of succinonitrile by reacting strong bases with weak acids such as Sodium or acrylonitrile (vinyl cyanide) with a ketone cy. potassium carbonates, cyanides, acetates, propi anohydrin. - onates, benzoates, etc. However, other basic mar Acrylonitrile is a well known cyanoethylating terials including primary and secondary amines 'agent reacting with the active hydrogen of an such as ethyl amine, dimethyl amine, ethylene OH group of many organic compounds to pro diamine, diethylene triamine, cyclohexyl amine, “duce the corresponding cyanoethyl derivatives. benzyl amine, ethanol amine, aniline, etc. are For example, aldehyde cyanohydrins such as also suitable, it being understood that basic ma glyconitrile and lactonitrile react with acrylo terials in general are operative. The amount of nitrile to form cyanoethyl derivatives. Accord basic catalyst used is likewise not a critical factor ingly, it was to be expected that reaction between and may be varied considerably with the ma acrylonitrile and a ketone cyanohydrin would also terial used but in general from 0.5 to 5% of cata result in cyanoethylation of the hydroxyl group 5 lyst based on the total Weight of the reactants is according to the following equation: employed.