By Mirabai R. Mccarthy the Climate and Structural Complexity of Tropical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Staghorn Fern - Platycerium Bifurcatum Platycerium Bifurcatum Is an Amazing Fern That Is Native to Eastern Australia
Staghorn Fern - Platycerium bifurcatum Platycerium bifurcatum is an amazing fern that is native to eastern Australia. It is one of eighteen species in the Platycerium genus, all of whom share a very dramatic, sculptural style. At first glance, most observers would not recognize these plants as ferns at all, since they are anything but ferny! Instead, the fronds of these beautiful, silvery green stunners resemble the antlers of elk or deer, which is why they have earned the common name of Staghorn or Elkhorn Fern. The resemblance is only heightened by the fact that they are epiphytes and grow outwards as if a large buck had left his rack hanging there. Platycerium bifurctum can easily be grown outdoors in subtropical gardens, but here in St. Louis we can imitate their native environment by mounting them on wooden plaques that can be brought indoors once the temperatures begin to cool. These plaques make striking decorations for a porch or patio. Learn how to craft your own on the next page. a few words on the anatomy of a staghorn • Staghorn ferns are epiphytes, clinging and growing vertically on tall trees or rock surfaces. They derive moisture and nutrients from the air and rain, supplemented by the plant debris that accumulates around their anchoring structures. • While the anchors for most epiphytes (such as orchids and bromeliads) are aerial roots or rhizomes, staghorn ferns add a covering layer of thick, spongy fronds that make a basket or inverted plate-like structure over the short, creeping rhizomes, providing a rooting media for the arching foliage fronds. -

Spores of Serpocaulon (Polypodiaceae): Morphometric and Phylogenetic Analyses
Grana, 2016 http://dx.doi.org/10.1080/00173134.2016.1184307 Spores of Serpocaulon (Polypodiaceae): morphometric and phylogenetic analyses VALENTINA RAMÍREZ-VALENCIA1,2 & DAVID SANÍN 3 1Smithsonian Tropical Research Institute, Center of Tropical Paleocology and Arqueology, Grupo de Investigación en Agroecosistemas y Conservación de Bosques Amazonicos-GAIA, Ancón Panamá, Republic of Panama, 2Laboratorio de Palinología y Paleoecología Tropical, Departamento de Ciencias Biológicas, Universidad de los Andes, Bogotá, Colombia, 3Facultad de Ciencias Básicas, Universidad de la Amazonia, Florencia Caquetá, Colombia Abstract The morphometry and sculpture pattern of Serpocaulon spores was studied in a phylogenetic context. The species studied were those used in a published phylogenetic analysis based on chloroplast DNA regions. Four additional Polypodiaceae species were examined for comparative purposes. We used scanning electron microscopy to image 580 specimens of spores from 29 species of the 48 recognised taxa. Four discrete and ten continuous characters were scored for each species and optimised on to the previously published molecular tree. Canonical correspondence analysis (CCA) showed that verrucae width/verrucae length and verrucae width/spore length index and outline were the most important morphological characters. The first two axes explain, respectively, 56.3% and 20.5% of the total variance. Regular depressed and irregular prominent verrucae were present in derived species. However, the morphology does not support any molecular clades. According to our analyses, the evolutionary pathway of the ornamentation of the spores is represented by depressed irregularly verrucae to folded perispore to depressed regular verrucae to irregularly prominent verrucae. Keywords: character evolution, ferns, eupolypods I, canonical correspondence analysis useful in phylogenetic analyses of several other Serpocaulon is a fern genus restricted to the tropics groups of ferns (Wagner 1974; Pryer et al. -

ABC Botanica 2-17.Indd
ACTA BIOLOGICA CRACOVIENSIA Series Botanica 59/2: 17–30, 2017 DOI: 10.1515/abcsb-2017-0011 POLSKA AKADEMIA NAUK ODDZIAŁ W KRAKOWIE A LOW RATIO OF RED/FAR-RED IN THE LIGHT SPECTRUM ACCELERATES SENESCENCE IN NEST LEAVES OF PLATYCERIUM BIFURCATUM JAKUB OLIWA, ANDRZEJ KORNAS* AND ANDRZEJ SKOCZOWSKI** Institute of Biology, Pedagogical University of Cracow, Podchorążych 2, 30-084 Kraków, Poland Received June 10, 2017; revision accepted September 18, 2017 The fern Platycerium bifurcatum is a valuable component of the flora of tropical forests, where degradation of local ecosystems and changes in lighting conditions occur due to the increasing anthropogenic pressure. In ferns, phytochrome mechanism responsible for the response to changes in the value of R/FR differs from the mechanism observed in spermatophytes. This study analyzed the course of ontogenesis of nest leaves in P. bifurcatum at two values of the R/FR ratio, corresponding to shadow conditions (low R/FR) and intense insolation (high R/FR). The work used only non-destructive research analysis, such as measurements of reflectance of radiation from the leaves, their blue-green and red fluorescence, and the chlorophyll a fluorescence kinetics. This allowed tracing the development and aging processes in the same leaves. Nest leaves are characterized by short, intense growth and rapid senescence. The study identified four stages of development of the studied leaves related to morphological and anatomical structure and changing photochemical efficiency of PSII. Under the high R/FR ratio, the rate of ontogenesis of the leaf lamina was much slower than under the low R/FR value. As shown, the rapid aging of the leaves was correlated with faster decline of the chlorophyll content. -

Quercus ×Coutinhoi Samp. Discovered in Australia Charlie Buttigieg
XXX International Oaks The Journal of the International Oak Society …the hybrid oak that time forgot, oak-rod baskets, pros and cons of grafting… Issue No. 25/ 2014 / ISSN 1941-2061 1 International Oaks The Journal of the International Oak Society … the hybrid oak that time forgot, oak-rod baskets, pros and cons of grafting… Issue No. 25/ 2014 / ISSN 1941-2061 International Oak Society Officers and Board of Directors 2012-2015 Officers President Béatrice Chassé (France) Vice-President Charles Snyers d’Attenhoven (Belgium) Secretary Gert Fortgens (The Netherlands) Treasurer James E. Hitz (USA) Board of Directors Editorial Committee Membership Director Chairman Emily Griswold (USA) Béatrice Chassé Tour Director Members Shaun Haddock (France) Roderick Cameron International Oaks Allen Coombes Editor Béatrice Chassé Shaun Haddock Co-Editor Allen Coombes (Mexico) Eike Jablonski (Luxemburg) Oak News & Notes Ryan Russell Editor Ryan Russell (USA) Charles Snyers d’Attenhoven International Editor Roderick Cameron (Uruguay) Website Administrator Charles Snyers d’Attenhoven For contributions to International Oaks contact Béatrice Chassé [email protected] or [email protected] 0033553621353 Les Pouyouleix 24800 St.-Jory-de-Chalais France Author’s guidelines for submissions can be found at http://www.internationaloaksociety.org/content/author-guidelines-journal-ios © 2014 International Oak Society Text, figures, and photographs © of individual authors and photographers. Graphic design: Marie-Paule Thuaud / www.lecentrecreatifducoin.com Photos. Cover: Charles Snyers d’Attenhoven (Quercus macrocalyx Hickel & A. Camus); p. 6: Charles Snyers d’Attenhoven (Q. oxyodon Miq.); p. 7: Béatrice Chassé (Q. acerifolia (E.J. Palmer) Stoynoff & W. J. Hess); p. 9: Eike Jablonski (Q. ithaburensis subsp. -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -

Growth of Fern Gametophytes After 20 Years of Storage in Liquid Nitrogen
FERN GAZ. 20(8): 337-346. 2018 337 GROWTH OF FERN GAMETOPHYTES AFTER 20 YEARS OF STORAGE IN LIQUID NITROGEN V. C. Pence Center for Conservation and Research of Endangered Wildlife (CREW) Cincinnati Zoo & Botanical Garden, 3400 Vine Street, Cincinnati, OH 45220, USA email: [email protected] Key words: cryopreservation, ex situ conservation, gametophyte; in vitro; long-term storage ABSTRACT In vitro grown gametophytes of six species of ferns, which had been cryopreserved using the encapsulation dehydration procedure, were evaluated for survival after 20 yrs of storage in liquid nitrogen. Tissues were rewarmed and transferred to a recovery medium with the same methods originally used to test pre-storage viability. All six species resumed growth. Post-storage viability was not consistently higher or lower than pre-storage viability of LN exposed tissues, likely reflecting the small sample sizes. However, these results demonstrate that long-term storage in liquid nitrogen is a viable option for preserving gametophytes of at least some fern species and could be utilized as an additional tool for preserving valuable gametophyte collections and for the ex situ conservation of fern biodiversity. INTRODUCTION For many species of ferns, gametophyte tissues have proven to be highly adaptable to growth in vitro (Table 1) . Most of these have been initiated through the aseptic germination of spores, although the aseptic germination of gemmae has also been demonstrated (Raine & Sheffield, 1997). As in vitro cultures, gametophytes can provide tissues for research and for propagation, both for ornamental ferns as well as for ferns of conservation concern. The ex situ conservation of ferns has traditionally relied on living collections and spore banks (Ballesteros, 2011). -

Morphological and Anatomical Adaptations to Dry, Shady Environments in Adiantum Reniforme Var
Morphological and anatomical adaptations to dry, shady environments in Adiantum reniforme var. sinense (Pteridaceae) Di Wu1, Linbao Li1, Xiaobo Ma1, Guiyun Huang1 and Chaodong Yang2 1 Rare Plants Research Institute of Yangtze River, Three Gorges Corporation, Yichang, China 2 Engineering Research Center of Ecology and Agriculture Use of Wetland, Ministry of Education, Yangtze University, Jingzhou, China ABSTRACT The natural distribution of the rare perennial fern Adiantum reniforme var. sinense (Pteridaceae), which is endemic to shady cliff environments, is limited to small areas of Wanzhou County, Chongqing, China. In this study, we used brightfield and epifluorescence microscopy to investigate the anatomical structures and histochemical features that may allow this species to thrive in shady, dry cliff environments. The A. reniforme var. sinense sporophyte had a primary structure and a dictyostele. The plants of this species had an endodermis, sclerenchyma layers and hypodermal sterome, reflecting an adaption to dry cliff environments. Blades had a thin cuticle and isolateral mesophyll, suggesting a tolerance of shady environments. These characteristics are similar to many sciophyte ferns such as Lygodium japonicum and Pteris multifida. Thus, the morphological and anatomical characteristics of A. reniforme var. sinense identified in this study are consistent with adaptations to shady, dry cliff environments. Subjects Conservation Biology, Plant Science Keywords Endodermis, Dictyostele, Sclerenchyma layer, Suberin lamellae, Thin cuticle Submitted 14 April 2020 Accepted 24 August 2020 INTRODUCTION Published 30 September 2020 Adiantum reniforme var. sinense (Pteridaceae, subfamily Vittarioideae) is a rare Corresponding authors Guiyun Huang, cliff-dwelling perennial pteridophyte, with a natural distribution limited to small areas of [email protected] Wanzhou County, Chongqing, China. -

11-122. 2000 11
FERN GAZ. 16(1, 2)11-122. 2000 11 CHECKLIST OF THE PTERIDOPHYTES OF TRINIDAD & TOBAGO Y. S. BAKSH-COMEAU The National Herbarium of Trinidad and Tobago. Department of Life Sciences, The University of the West Indies, St. Augustine, Trinidad, West Indies Key words: checklist, Trinidad and Tobago pteridophytes, types, habitat, distribution. ABSTRACT Three hundred and two species and eight varieties or subspecies in 27 families and 77 genera of ferns and fern allies are listed. Four new combinations and states are made, and one synonym lectotypified. A serious attempt has been made to establish types; selections of specimens studied are cited. INTRODUCTION Recent studies of ferns in Trinidad and Tobago (Baksh-Comeau, 1996, 1999) have combined a review of the pteridophyte collection at The National Herbarium of Trinidad & Tobago with field surveys undertaken to assess the community status of these plants on both islands. This checklist has been developed as an integral part of those studies, but it is also an essential prerequisite to ongoing research covering a reclassification of the vegetation of the islands and to the preparation of a comprehensive vascular plant flora. The herbarium count and field survey revealed 251 species confirmed by voucher specimens housed in Trinidad. Additional species have been attributed to Trinidad or Tobago in early publications for Trinidad and in Floras and monographs for neighbouring areas. The number of species now believed to be indigenous in these islands is 282. Cultivated species that have escaped, and introductions which have become naturalized number 20. Early reports include Grisebach (1859-64) who listed 106 species; Eaton (1878) approximately 78 of the 150 or so species eventually collected by August Fendler; Jenman (1887) had about 184 species; Anon (1889) listed 206 binomials including a few introduced taxa; Jenman (1898-1909), in an incomplete coverage of the fern flora, described 140 taxa of which 10 were new species; Hart (1908), including some cultivated plants, listed 283 binomials of pteridophytes. -

Pleopeltis ×Cerro-Altoensis (Polypodiaceae), a New Fern Hybrid from Robinson Crusoe Island (Juan Fernandez Archipelago, Chile)
FERN GAZ. 20(2):65-78. 2015 65 PLEOPELTIS ×CERRO-ALTOENSIS (POLYPODIACEAE), A NEW FERN HYBRID FROM ROBINSON CRUSOE ISLAND (JUAN FERNANDEZ ARCHIPELAGO, CHILE) P. DANTON 1*, M. BOUDRIE 2, A. BIZOT 3 & R.L.L. VIANE 4 15, rue Galilée, F-38000 Grenoble, France. E-Mail: 216, rue des Arènes, F-87000 Limoges, France. E-mail: 3 1, rue de la Faye, F-08160 Hannogne-Saint-Martin, France . E-mail: [email protected] 4 Universiteit Ghent, Vakgroep Biologie, Pteridologie, K.L. Ledeganckstraat 35, Bm-9ic0h0e0l bGohuednrt,i eB@elogriuamng. e.fr E-mail: * Auathronra ufodr. bciozroret@spwonadneandcoe o.fr Keywords : Pleopeltis , hybrid, Polypodiaceae, Juan Fernández, Chile [email protected] ABSTRACT A fern hybrid of the genus Pleopeltis was discovered on Robinson Crusoe Island in the Juan Fernández Archipelago, off the coast of Chile, and is described as P. ×cerro-altoensis . Its putative parents are P. macrocarpa and P. masafuerae , two species present in the archipelago. Mots-clés : Pleopeltis , hybride, Polypodiaceae, Juan Fernández, Chili RÉSUMÉ Un hybride de fougère appartenant au genre Pleopeltis a été découvert sur l’île Robinson Crusoë, dans l’archipel Juan Fernández, au large du Chili, et est décrit sous le nom de P. × cerro-altoensis . Ses parents probables sont P. macrocarpa et P. masafuerae , deux espèces présentes dans l’archipel. Palabras clavas : Pleopeltis , híbrido, Polypodiaceae, Juan Fernández, Chile RESUMEN Un híbrido de helecho que pertenece al género Pleopeltis ha sido descubierto en la isla Robinson Crusoe, en el archipiélago Juan Fernández, a la altura de Chile, y es descrito con el nombre de P. -
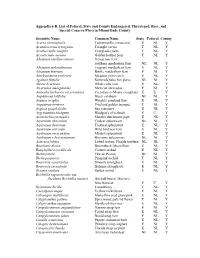
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

Epiphytes and the National Wetland Plant List
Lichvar, R.W. and W. Fertig. 2011. Epiphytes and the National Wetland Plant List. Phytoneuron 2011-16: 1–31. EPIPHYTES AND THE NATIONAL WETLAND PLANT LIST ROBERT W. LICHVAR U.S. Army Engineer Research and Development Center Cold Regions Research and Engineering Laboratory 72 Lyme Road Hanover, NH 03755-1290 WALTER FERTIG Moenave Botanical Consulting 1117 West Grand Canyon Drive Kanab, UT 84741 ABSTRACT The National Wetland Plant List (NWPL) is a list of species that occur in wetlands in the United States. It is a product of a collaborative effort of four Federal agencies: the U.S. Army Corps of Engineers, the U.S. Environmental Protection Agency, the U.S. Fish and Wildlife Service, and the Natural Resources Conservation Service. The NWPL has many uses, but it is specifically designed for use in wetland delineation for establishing the extent of Federal jurisdictional of wetland boundaries. To be listed in the NWPL, a plant must be rooted in soil, so there is a direct relationship between a plant’s occurrence and its preference for hydric soils. This relationship, coupled with the plant’s frequency of occurrence in wetlands, is used to place it in one of five categories representing the probability that the plant occurs in a wetland. Many species are considered to be epiphytes, but they represent various life forms, ranging from purely epiphytic to frequently occurring on the ground. Based on a literature review of 192 species across the United States and its territories, we determined which species fell into four categories of epiphytic life forms or are terrestrial and should not be considered epiphytes.