Genetic Variations of Lansium Domesticum Corr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mindoro East Coast Road Project
E1467 v 5 Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Table of Contents l'age I Executive Summary 1 I1 Project Description 4 Project Ra.tionale 4 Basic Project Information 5 Project Location 5 Description of Project Phases 6 111 Methodology Existing Erivironmental Condition Physical Environment Biological Environment Socio-Economic Environment IV Impact Assessment 23 Future Environmental Condition of the Project Area 23 Impacts Relating to Project Location 24 Impacts Relating to Project Construction 26 lmpacts Relating to Project Operation and Maintenance 30 V Environmental Management Plan 31 Environmental Monitoring Plan 39 VI ANNEXES Location Map Photographs along the Project Road Typical Section for flexible and rigid pavement Typical section of Bridge superstructure Provincial & Municipal Resolution Accountab~lityStatements Executive Summary Initial Environmental Examination (IEE) Mindoro East Coast Road Proiect Executive Summary A. Introduction The Environmental Impact Assessment (EIA) of the proposed Rehabilitationllmprovement of Mindoro East Coast Road Project (Bongabong - Roxas - Mansalay - Bulalacao - Magsaysay - San Jose Section) is presented in the form of an Initial Environmental Examination (IEE) to secure an Environmental Compliance Certificate (ECC) in accordance with the requirement of the revised rules and regulations of the Environmental Impact Statement System (EISS) embodied in .the Department of Environment and Natural Resources - Department Administrative Order (DENR-DAO) 96-37 Thus, this report covers the result of the said EIA that aims to confirm the environmental viability of implementing the proposed project. B. Project Description The 125.66 kilonieter Mindoro East Coast Road Project traverses the two provinces in the Island of Mindoro. It passes thru the municipalities of Bongabong, Roxas, Mansalay and Bulalacao in Oriental Mindoro and Magsaysay and San Jose in Occidental Mindoro. -

Philippine Drug Enforcement Agency
Republic of the Philippines Office of the President PHILIPPINE DRUG ENFORCEMENT AGENCY Regional Office IV-B (MIMAROPA) Unit 14 Filipiniana Complex, Calapan City, Oriental Mindoro 5200 | www.pdea.gov.ph | [email protected] | (043) 441-0267 MONTHLY REGIONAL WEBPAGE UPDATE I. SIGNIFICANT OPERATIONAL ACCOMPLISHMENTS The following are the anti-illegal drug operations conducted by this Office and other law enforcement units that resulted in the arrests of High Value Targets (HVTs) for the month of July 1-31, 2018: Barangay Chairman caught for possessing shabu A Barangay Chairman was arrested in Search and Seizure operation at Brgy. Maragooc, Gloria, Oriental Mindoro. Suspect was identified as Domingo Mingo Mortel, Filipino, 50 years old, male, married, Barangay Chairman and a resident of Brgy. Maragooc, Gloria, Oriental Mindoro. That on 7th July 2018 at 0600H, joint elements of PDEA Oriental Mindoro Provincial Office, Gloria MPS and PNP Maritime Group 4B-02 implemented a search warrant at Brgy. Maragooc, Gloria, Oriental Mindoro, which resulted in the arrest of Brgy. Captain Domingo Mingo Mortel. Confiscated during the search were two (2) pieces heat sealed transparent plastic sachets of Methamphetamine Hydrochloride known as shabu weighing 0.0151 gram and one (1) unit caliber 45 Armscor pistol. Cases for violation of Section 11 Article II of RA9165 and RA 10591 were filed against the suspect. # # # # # Notorious member of a drug group busted in an entrapment operation A member of Garcia Drug Group was arrested in buy-bust operation at Sitio Roma Sur, Brgy. Roma, Mansalay, Oriental Mindoro. Suspect was identified as Arnel Olivas Morillo, Filipino, 50 years old, male, married, jobless and a resident of Brgy. -
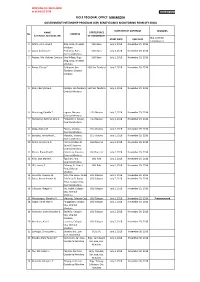
Dole Regional Office Mimaropa Government Internship Program (Gip) Beneficiaries Monitoring Form (Fy 2018)
PROFILING OF CHILD LABOR as of July 25, 2018 DOLE-GIP_Form C DOLE REGIONAL OFFICE MIMAROPA GOVERNMENT INTERNSHIP PROGRAM (GIP) BENEFICIARIES MONITORING FORM (FY 2018) DURATION OF CONTRACT REMARKS NAME OFFICE/PLACE No. ADDRESS (Last Name, First Name, MI) OF ASSIGNMENT (e.g. Contract START DATE END DATE completed or 1 Alforo, John Lloyd Z. Alag, Baco, Oriental LGU Baco July 2, 2018 November 29, 2018 Mindoro 2 Lapat, Anthony O. Poblacion, Baco, LGU Baco July 2, 2018 November 29, 2018 Oriental Mindoro 3 Nebres, Ma. Dolores Corazon A.Sitio Hilltop, Brgy. LGU Baco July 2, 2018 November 29, 2018 Alag, Baco, Oriental Mindoro 4 Rance, Elaesa E. Poblacion, San LGU San Teodoro July 2, 2018 November 29, 2018 Teodoro, Oriental Mindoro 5 Rizo, CherryMae A. Calsapa, San Teodoro, LGU San Teodoro July 2, 2018 November 29, 2018 Oriental Mindoro 6 Macarang, Cybelle T. Laguna, Naujan, LGU Naujan July 2, 2018 November 29, 2018 Oriental Mindoro 7 Mantaring, Kathryn Jane A. Poblacion II, Naujan, LGU Naujan July 2, 2018 November 29, 2018 Oriental Mindoro 8 Abog, Orpha M. Pakyas, Victoria, LGU Victoria July 2, 2018 November 29, 2018 Oriental Mindoro 9 Boncato, Jenna Mae C. Macatoc, Victoria, LGU Victoria July 2, 2018 November 29, 2018 Oriental Mindoro 10 Nefiel, Jeric John D. Flores de Mayo St. LGU Socorro July 2, 2018 November 29, 2018 Zone IV, Socorro, Oriental Mindoro 11 Platon, Bryan Paul R. Calocmoy, Socorro, LGU Socorro July 2, 2018 November 29, 2018 Oriental Mindoro 12 Nillo, Joza Marie D. Tiguihan, Pola, LGU Pola July 2, 2018 November 29, 2018 Oriental Mindoro 13 Ulit, Lovely E. -

One Big File
MISSING TARGETS An alternative MDG midterm report NOVEMBER 2007 Missing Targets: An Alternative MDG Midterm Report Social Watch Philippines 2007 Report Copyright 2007 ISSN: 1656-9490 2007 Report Team Isagani R. Serrano, Editor Rene R. Raya, Co-editor Janet R. Carandang, Coordinator Maria Luz R. Anigan, Research Associate Nadja B. Ginete, Research Assistant Rebecca S. Gaddi, Gender Specialist Paul Escober, Data Analyst Joann M. Divinagracia, Data Analyst Lourdes Fernandez, Copy Editor Nanie Gonzales, Lay-out Artist Benjo Laygo, Cover Design Contributors Isagani R. Serrano Ma. Victoria R. Raquiza Rene R. Raya Merci L. Fabros Jonathan D. Ronquillo Rachel O. Morala Jessica Dator-Bercilla Victoria Tauli Corpuz Eduardo Gonzalez Shubert L. Ciencia Magdalena C. Monge Dante O. Bismonte Emilio Paz Roy Layoza Gay D. Defiesta Joseph Gloria This book was made possible with full support of Oxfam Novib. Printed in the Philippines CO N T EN T S Key to Acronyms .............................................................................................................................................................................................................................................................................. iv Foreword.................................................................................................................................................................................................................................................................................................... vii The MDGs and Social Watch -

2019 Annual Regional Economic Situationer
2019 ANNUAL REGIONAL ECONOMIC SITUATIONER National Economic and Development Authority MIMAROPA Region Republic of the Philippines National Economic and Development Authority MIMAROPA Region Tel (43) 288-1115 E-mail: [email protected] Fax (43) 288-1124 Website: mimaropa.neda.gov.ph ANNUAL REGIONAL ECONOMIC SITUATIONER 2019 I. Macroeconomy A. 2018 Gross Regional Domestic Product (GRDP) Among the 17 regions of the country, MIMAROPA ranked 2nd— together with Davao Region and next to Bicol Region—in terms of growth rate. Among the major economic sectors, the Industry sector recorded the fastest growth of 11.2 percent in 2018 from 1.6 percent in 2017. This was followed by the Services sector, which grew by 9.3 percent in 2018 from 8.7 percent in 2017. The Agriculture, Hunting, Fishery and Forestry (AHFF) sector also grew, but at a slower pace at 2.6 percent in 2018 from 3.0 percent in 2017 (refer to Table 1). Table 1. Economic Performance by Sector and Subsector, MIMAROPA, 2017-2018 (at constant 2000 prices, in percent except GVA) Contribution Percent 2017 2018 GRDP Growth rate Sector/Subsector GVA GVA distribution growth (in P '000) (in P '000) 2017 2018 17-18 16-17 17-18 Agriculture, hunting, 26,733,849 27,416,774 20.24 19.12 0.5 3.0 2.6 forestry, and fishing Agriculture and 21,056,140 21,704,747 15.94 15.13 0.5 4.4 3.1 forestry Fishing 5,677,709 5,712,027 4.30 3.98 0.0 -1.9 0.6 Industry sector 42,649,103 47,445,680 32.29 33.08 3.7 1.6 11.2 Mining and 23,830,735 25,179,054 18.04 17.56 1.0 -5.5 5.7 quarrying Manufacturing 6,811,537 7,304,895 -

Cbmspovertymaps Vol2 Orient
The Many Faces of Poverty Volume 2 The Many Faces of Poverty: Volume 2 Copyright © PEP-CBMS Network Office, 2011 ALL RIGHTS RESERVED. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means—whether virtual, electronic, mechanical, photocopying, recording, or otherwise—without the written permission of the copyright owner. Acknowledgements The publication of this volume has been made possible through the PEP- CBMS Network Office based at the Angelo King Institute for Economic and Business Studies of De La Salle University-Manila with the aid of a grant from the International Development Research Centre (IDRC), Ottawa, Canada and the Canadian International Development Agency (CIDA). CONTENTSCONTENTS i Foreword 1 Introduction 3 Explanatory Text The Many Faces of Poverty 9 Agusan del Sur 61 Dinagat Islands 95 Marinduque 139 Oriental Mindoro 201 Palawan 247 Sarangani 281 Southern Leyte FOREWORDFOREWORD The official poverty monitoring system (PMS) in the Philippines relies mainly on family income and expenditure surveys. Information on other aspects of well-being is generally obtained from representative health surveys, national population and housing censuses, and others. However, these surveys and censuses are (i) too costly to be replicated frequently; (ii) conducted at different time periods, making it impossible to get a comprehensive profile of the different socio-demographic groups of interest at a specific point in time; and (iii) have sampling designs that do not usually correspond to the geographical disaggregation needed by local government units (LGUs). In addition, the implementation of the decentralization policy, which devolves to LGUs the function of delivering basic services, creates greater demand for data at the local level. -

Region Penro Cenro Province Municipality Barangay
REGION PENRO CENRO PROVINCE MUNICIPALITY BARANGAY DISTRICT AREA IN HECTARES NAMEOF ORGANIZATION TYPE OF ORGANIZATION COMPONENT COMMODITY SPECIES YEAR ZONE TENURE RIVER BASIN NUMBER OF LOA WATERSHED SITECODE REMARKS MIMAROPA Marinduque Boac Marinduque Buenavista Sihi Lone District 34.02 LGU-Sihi LGU Reforestation Timber Narra 2011 Protection 11-174001-0001-0034 MIMAROPA Marinduque Boac Marinduque Boac Tumagabok Lone District 8.04 LGU-Tumagabok LGU Agroforestry Timber and Fruit Trees Narra, Langka, Guyabano, and Rambutan 2011 Production 11-174001-0002-0008 MIMAROPA Marinduque Boac Marinduque Torrijos Sibuyao Lone District 2.00 LGU-Sibuyao LGU Agroforestry Fruit Trees Langka 2011 Production 11-174001-0003-0002 MIMAROPA Marinduque Boac Marinduque Torrijos Sibuyao Lone District 12.01 LGU-Sibuyao LGU Reforestation Timber Narra 2011 Protection Untenured 11-174001-0004-0012 MIMAROPA Marinduque Boac Marinduque Torrijos Sibuyao Lone District 7.04 LGU-Sibuyao LGU Reforestation Timber Narra 2011 Protection 11-174001-0005-0007 MIMAROPA Marinduque Boac Marinduque Torrijos Sibuyao Lone District 3.00 LGU-Sibuyao LGU Reforestation Timber Narra 2011 Protection 11-174001-0006-0003 MIMAROPA Marinduque Boac Marinduque Torrijos Sibuyao Lone District 1.05 LGU-Sibuyao LGU Reforestation Timber Narra 2011 Protection 11-174001-0007-0001 MIMAROPA Marinduque Boac Marinduque Torrijos Sibuyao Lone District 2.03 LGU-Sibuyao LGU Reforestation Timber Narra 2011 Protection 11-174001-0008-0002 MIMAROPA Marinduque Boac Marinduque Buenavista Yook Lone District 30.02 LGU-Yook -

31 October 2020
31 October 2020 At 5:00 AM, TY "ROLLY" maintains its strength as it moves closer towards Bicol Region. The eye of Typhoon "ROLLY" was located based on all available data at 655 km East Northeast of Virac, Catanduanes. TCWS No. 2 was raised over Catanduanes, the eastern portion of Camarines Sur, Albay, and Sorsogon. While TCWS No.1 was raised over Camarines Norte, the rest of Camarines Sur, Masbate including Ticao and Burias Islands, Quezon including Polillo Islands, Rizal, Laguna, Cavite, Batangas, Marinduque, Romblon, Occidental Mindoro including Lubang Island, Oriental Mindoro, Metro Manila, Bulacan, Pampanga, Bataan, Zambales, Tarlac, Nueva Ecija, Aurora, Pangasinan, Benguet, Ifugao, Nueva Vizcaya, Quirino, and the southern portion of Isabela, Northern Samar, the northern portion of Samar, the northern portion of Eastern Samar, and the northern portion of Biliran. At 7:00 PM, the eye of TY "ROLLY" was located based on all available data at 280 km East Northeast of Virac, Catanduanes. "ROLLY" maintains its strength as it threatens Bicol Region. The center of the eye of the typhoon is likely to make landfall over Catanduanes early morning of 01 November 2020, then it will pass over mainland Camarines Provinces tomorrow morning, and over mainland Quezon tomorrow afternoon. At 10:00 PM, the eye of TY "ROLLY" was located based on all available data including those from Virac and Daet Doppler Weather Radars at 185 km East of Virac, Catanduanes. Bicol Region is now under serious threat as TY "ROLLY" continues to move closer towards Catanduanes. Violent winds and intense to torrential rainfall associated with the inner rainband-eyewall region will be experienced over (1) Catanduanes tonight through morning; (2) Camarines Provinces and the northern portion of Albay including Rapu-Rapu Islands tomorrow early morning through afternoon. -

Oriental Mindoro Facts and Figures 2013 Table of Contents
ORIENTAL MINDORO FACTS AND FIGURES 2013 TABLE OF CONTENTS Page General Information 1 Administrative Map 2 Land and Other Natural Resources 3 a. Land Area by Municipality 3 b. Land Classification Statistics 3 c. Geographical Zone Surfaces 3 d. Mineral Resources 4 e. Forest Cover 4 f. Coastal Resources 5 Demography 8 a. Population Size by Municipality by Census 8 Years b. Actual and Projected Population and Number 9 of Households, Growth Rate by Municipality c. Life Expectancy 9 d. Projected Population by Province, MIMAROPA 10 e. Urban-Rural Population 10 f. Population Density 11 g. Mangyan Tribes by Municipality 11 h. Mangyan Households by Sex 12 Economic Profile 13 a. Agriculture 13 b. Tourism 18 c. Commerce and Industry 22 Infrastructure and Utilities 24 a. Transportation 24 b. Communication 25 c. Water 27 d. Power 28 Social Development Profile 30 a. Labor and Employment 30 b. Poverty and Income 30 c. Health 33 d. Education 36 e. Social Welfare Services 37 f. Protective Services 37 Financial Profile 39 a. Income Classification of City/Municipality 39 b. Annual Income and Budget Per 39 City/Municipality c. Income and Expenditure, Provincial 40 Government f Oriental Mindoro Institutional Profile 41 a. Organizational Chart of the Provincial 41 Government of Oriental Mindoro b. Provincial Government Personnel by Office 42 ORIENTAL MINDORO FACTS AND FIGURES 2013 General Information A. LOCATION Oriental Mindoro is located in Region IV-B, otherwise known as the MIMAROPA Region. It lies 45 kilometers south of Batangas and 130 kilometers south of Manila. B. BOUNDARY It is bounded on the North by Verde Island Passage; Maestro del Campo Island and Tablas Strait on the East; Semirara Island on the South; and Occidental Mindoro on the West. -
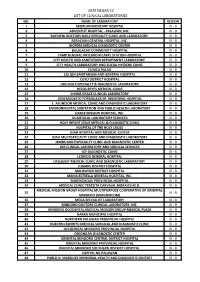
2020 Neqas-Cc List of Clinical Laboratories No
2020 NEQAS-CC LIST OF CLINICAL LABORATORIES NO. NAME OF LABORATORY REGION 1 ABORLAN MEDICARE HOSPITAL IV - B 2 ADVENTIST HOSPITAL - PALAWAN, INC. IV - B 3 BAYVIEW DOCTORS MULTISPECIALTY CLINIC AND LABORATORY IV - B 4 BERACHAH GENERAL HOSPITAL, INC. IV - B 5 BIOFERA MEDICAL DIAGNOSTIC CENTER IV - B 6 BULALACAO COMMUNITY HOSPITAL IV - B 7 CAMP GENERAL ARTEMIO RICARTE STATION HOSPITAL IV - B 8 CITY HEALTH AND SANITATION DEPARTMENT LABORATORY IV - B 9 CITY HEALTH LABORATORY AND SOCIAL HYGIENE CLINIC IV - B 10 CLINICA PALAO IV - B 11 CULION SANITARIUM AND GENERAL HOSPITAL IV - B 12 CUYO DISTRICT HOSPITAL IV - B 13 DBS MULTISPECIALTY & DIAGNOSTIC LABORATORY IV - B 14 DELOS REYES MEDICAL CLINIC IV - B 15 DIVINE GRACE CLINICAL LABORATORY IV - B 16 DON MODESTO FORMILLEZA SR. MEMORIAL HOSPITAL IV - B 17 E. ASUNCION MEDICAL CLINIC AND DIAGNOSTIC LABORATORY IV - B 18 ENVIRONMENTAL SANITATION AND PUBLIC HEALTH LABORATORY IV - B 19 GRACE MISSION HOSPITAL, INC. IV - B 20 GS MEDICAL LABORATORY SERVICES IV - B 21 HOLY INFANT JESUS MEDICAL & DIAGNOSTIC CLINIC IV - B 22 HOSPITAL OF THE HOLY CROSS IV - B 23 ISIAH HOSPITAL AND MEDICAL CENTER IV - B 24 ISIAH MULTISPECIALTY CLINIC AND DIAGNOSTIC LABORATORY IV - B 25 JBMBS MULTISPECIALTY CLINIC AND DIAGNOSTIC CENTER IV - B 26 LBR CLINICAL LABORATORY AND MEDICAL SERVICES IV - B 27 LCF DIAGNOSTIC CLINIC IV - B 28 LEONCIO GENERAL HOSPITAL IV - B 29 LIFEQUEST MEDICAL CLINIC AND DIAGNOSTIC LABORATORY IV - B 30 LUBANG DISTRICT HOSPITAL IV - B 31 MALIPAYON DISTRICT HOSPITAL IV - B 32 MARIA ESTRELLA GENERAL HOSPITAL, INC. IV - B 33 MARINDUQUE PROVINCIAL HOSPITAL IV - B 34 MEDICAL CLINIC-TERESITA CARVAJAL MORALES M.D. -

Regional Report on the Approved Construction Safety & Health Program
REGIONAL REPORT ON THE APPROVED CONSTRUCTION SAFETY & HEALTH PROGRAM (CSHP) DOLE-MIMAROPA October 2017 Date No. Company Name and Address Project Name Project Address Approved PABLO S. LABASBAS 1 CONSTRUCTION/ Brgy. Dagum, Core Local Access Road/ Municipality of Odiongan Dapawan, Odiongan, Romblon 02-Oct-17 Calbayog City R.G. FLORENTINO CONSTRUCTION Repair/ Rehabilitation of San Andres District Hospital 2 Poblacio, San Andres, Romblon 02-Oct-17 AND TRADING/ San Andres, Romblon (Painting and Flooring)/ Province of Romblon CSR CONSTRUCTION AND SUPPLY/ Construction of San Fernando Multi-Purpose Building 3 San Fernando, Romblon 02-Oct-17 Sugod, Cajidiocan, Romblon Phase 12/ Province of Romblon CSR CONSTRUCTION AND SUPPLY/ Repair/ Rehabilitation of Pandan Bridge/ Province of 4 Sta. Fe, Romblon 02-Oct-17 Sugod, Cajidiocan, Romblon Romblon CSR CONSTRUCTION AND SUPPLY/ Slope Protection of Barangay Tabin-Dagat to Ligaya/ 5 Odiongan, Romblon 02-Oct-17 Sugod, Cajidiocan, Romblon Province of Romblon CSR CONSTRUCTION AND SUPPLY/ Concreting of Poblacion to Bachawan Road/ Province of 6 Concepcion, Romblon 02-Oct-17 Sugod, Cajidiocan, Romblon Romblon HARDSHELL DESIGN AND Construction of Amphitheater Building/ Palawan State PSU Main Campus, Puerto Princesa 7 CONSTRUCTION/ 67-D Burgos St., 03-Oct-17 University City, Palawan Brgy. Masikap, Puerto Princesa City HARDSHELL DESIGN AND Construction of CBA Business Enterprise Building/ PSU Main Campus, Puerto Princesa 8 CONSTRUCTION/ 67-D Burgos St., 03-Oct-17 Palawan State University City, Palawan Brgy. Masikap, Puerto Princesa City HARDSHELL DESIGN AND Construction of Two-Storey Administration Building, 9 CONSTRUCTION/ 67-D Burgos St., PSU Quezon Campus, Palawan 03-Oct-17 Phase II/ Palawan State University Brgy. -

3D Critical Areas
Philippine National Bank (Europe) Plc Door to Door Listing as of 13/10/2006 AREA DELIVERY CATEGORY REGION 4-B MARINDUQUE BOAC OSA BUENAVISTA OSA GASAN OSA MOGPOG OSA STA. CRUZ OSA TORRIJOS OSA MINDORO OCCIDENTAL ABRA DE ILOG OSA CALINTAAN OSA LOOC OSA LUBANG OSA MAGSAYSAY OSA MAMBURAO OSA PALUAN OSA RIZAL OSA SABLAYAN OSA STA. CRUZ OSA MINDORO ORIENTAL BACO S CALAPAN * canubing II OSA * gutad OSA * maidlang OSA * nag iba II OSA * navotas OSA * sta cruz OSA * selonai OSA NAUJAN * malayaOSA * pagkakaisa OSA * malinao OSA PUERTO GALERA OSA * talipanan OSA * aninuan OSA * white beach OSA * san isidro OSA * minolo OSA * balatero OSA * boquete OSA * san antonio OSA * poblacion OSA * dalaruan OSA * palangan OSA * sabang OSA * sinandigan OSA * tabinay OSA Legend: OTD - Out of Town Delivery OSA - Out of Service Area NS - Non-Serviceable Philippine National Bank (Europe) Plc Door to Door Listing as of 13/10/2006 * villaflor OSA * dulangan OSA SAN TEODORO OSA GLORIA * balete OSA * langlang OSA * tinalunan OSA * bulbugan OSA * banus OSA * lucio laurel OSA PINAMALAYAN * anoling OTD * bangbang OTD * bacungan OTD * guinhawa OTD * malaya OTD * maliancog OTD * pili OTD * sta. Isabel OTD * banilad OSA * buli OSA * inclanay OSA * marayos OSA * pambisan OSA * ranzon OSA * sabang OSA * simborio OSA * upper bongol OSA * lower bongol OSA POLA OSA BANSUD OSA BONGABONG OSA BULALACAO OSA MANSALAY OSA ROXAS OSA * poblacion OSA * cantil OSA SOCORRO * leuteboro 1 & 2 OTD PALAWAN ABORLAN * sagpangan OTD * tigman OTD * cabigaan OTD * barake OTD * marikit OTD * apo-aporawan