Β-Catenin: a Key Mediator of Wnt Signaling Karl Willert∗ and Roel Nusse²
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

REVIEW Signal Transduction, Cell Cycle Regulatory, and Anti
Leukemia (1999) 13, 1109–1166 1999 Stockton Press All rights reserved 0887-6924/99 $12.00 http://www.stockton-press.co.uk/leu REVIEW Signal transduction, cell cycle regulatory, and anti-apoptotic pathways regulated by IL-3 in hematopoietic cells: possible sites for intervention with anti-neoplastic drugs WL Blalock1, C Weinstein-Oppenheimer1,2, F Chang1, PE Hoyle1, X-Y Wang3, PA Algate4, RA Franklin1,5, SM Oberhaus1,5, LS Steelman1 and JA McCubrey1,5 1Department of Microbiology and Immunology, 5Leo Jenkins Cancer Center, East Carolina University School of Medicine Greenville, NC, USA; 2Escuela de Quı´mica y Farmacia, Facultad de Medicina, Universidad de Valparaiso, Valparaiso, Chile; 3Department of Laboratory Medicine and Pathology, Mayo Clinic and Foundation, Rochester, MN, USA; and 4Division of Basic Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA Over the past decade, there has been an exponential increase growth factor), Flt-L (the ligand for the flt2/3 receptor), erythro- in our knowledge of how cytokines regulate signal transduc- poietin (EPO), and others affect the growth and differentiation tion, cell cycle progression, differentiation and apoptosis. Research has focused on different biochemical and genetic of these early hematopoietic precursor cells into cells of the 1–4 aspects of these processes. Initially, cytokines were identified myeloid, lymphoid and erythroid lineages (Table 1). This by clonogenic assays and purified by biochemical techniques. review will concentrate on IL-3 since much of the knowledge This soon led to the molecular cloning of the genes encoding of how cytokines affect cell growth, signal transduction, and the cytokines and their cognate receptors. -

Loss of At-Catenin Alters the Hybrid Adhering Junctions in the Heart and Leads to Dilated Cardiomyopathy and Ventricular Arrhythmia Following Acute Ischemia
1058 Research Article Loss of aT-catenin alters the hybrid adhering junctions in the heart and leads to dilated cardiomyopathy and ventricular arrhythmia following acute ischemia Jifen Li1,*, Steven Goossens2,3,`, Jolanda van Hengel2,3,`, Erhe Gao1, Lan Cheng1, Koen Tyberghein2,3, Xiying Shang1, Riet De Rycke2, Frans van Roy2,3,* and Glenn L. Radice1 1Center for Translational Medicine, Department of Medicine, Thomas Jefferson University, Philadelphia, PA, USA 2Department for Molecular Biomedical Research, Flanders Institute for Biotechnology (VIB), B-9052 Ghent, Belgium 3Department of Biomedical Molecular Biology, Ghent University, B-9052 Ghent, Belgium *Authors for correspondence ([email protected]; [email protected]) `These authors contributed equally to this work Accepted 4 October 2011 Journal of Cell Science 125, 1058–1067 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.098640 Summary It is generally accepted that the intercalated disc (ICD) required for mechano-electrical coupling in the heart consists of three distinct junctional complexes: adherens junctions, desmosomes and gap junctions. However, recent morphological and molecular data indicate a mixing of adherens junctional and desmosomal components, resulting in a ‘hybrid adhering junction’ or ‘area composita’. The a-catenin family member aT-catenin, part of the N-cadherin–catenin adhesion complex in the heart, is the only a-catenin that interacts with the desmosomal protein plakophilin-2 (PKP2). Thus, it has been postulated that aT-catenin might serve as a molecular integrator of the two adhesion complexes in the area composita. To investigate the role of aT-catenin in the heart, gene targeting technology was used to delete the Ctnna3 gene, encoding aT-catenin, in the mouse. -

82135357.Pdf
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Developmental Biology 319 (2008) 298–308 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Identification of a novel intermediate filament-linked N-cadherin/γ-catenin complex involved in the establishment of the cytoarchitecture of differentiated lens fiber cells Michelle Leonard, Yim Chan, A. Sue Menko ⁎ Department of Pathology, Anatomy and Cell Biology, Thomas Jefferson University, 571 Jefferson Alumni Hall, 1020 Locust Street, Philadelphia, PA 19107, USA article info abstract Article history: Tissue morphogenesis and maintenance of complex tissue architecture requires a variety of cell–cell junctions. Received for publication 17 December 2007 Typically, cells adhere to one another through cadherin junctions, both adherens and desmosomal junctions, Revised 14 April 2008 strengthened by association with cytoskeletal networks during development. Both β-andγ-catenins are Accepted 18 April 2008 reported to link classical cadherins to the actin cytoskeleton, but only γ-catenin binds to the desmosomal Available online 8 May 2008 cadherins, which links them to intermediate filaments through its association with desmoplakin. Here we fi γ Keywords: provide the rst biochemical evidence that, in vivo, -catenin also mediates interactions between classical fi γ-catenin cadherins and the intermediate lament cytoskeleton, linked through desmoplakin. In the developing lens, Cadherin which has no desmosomes, we discovered that vimentin became linked to N-cadherin complexes in a Intermediate filament differentiation-state specific manner. This newly identified junctional complex was tissue specific but not Vimentin unique to the lens. To determine whether in this junction N-cadherin was linked to vimentin through γ- Lens development catenin or β-catenin we developed an innovative “double” immunoprecipitation technique. -

Overexpression of Vimentin Contributes to Prostate Cancer Invasion and Metastasis Via Src Regulation
ANTICANCER RESEARCH 28: 327-334 (2008) Overexpression of Vimentin Contributes to Prostate Cancer Invasion and Metastasis via Src Regulation JUNCHENG WEI*, GANG XU*, MINGFU WU, YONGTAO ZHANG, QIONG LI, PING LIU, TAO ZHU, ANPING SONG, LIANGPIN ZHAO, ZHIQIANG HAN, GANG CHEN, SHIXUAN WANG, LI MENG, JIANFENG ZHOU, YUNPING LU, SHIXUAN WANG and DING MA Cancer Biology Research Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, P.R. China Abstract. A significant proportion of prostate cancer patients Prostate carcinoma is the second leading cause of cancer- treated with curative intent develop advanced disease. At a related death in the United States and Europe (1), mainly fundamental biological level, very little is known about what due to its high potential for bone metastasis. However, the makes the disease aggressive and metastatic. Observational molecular mechanisms of prostate cancer metastasis are not pathology reports and experimental data suggest that an well understood. Among the currently available techniques, epithelial-mesenchymal transition (EMT) is involved in prostate cancer proteomics permits the analysis of thousands of cancer invasiveness. The mechanism by which vimentin modified or unmodified proteins simultaneously and has promotes prostate cancer cell invasion and metastasis was become increasingly popular for identifying biomarkers for examined. The highly metastatic human prostate epithelial cell the early detection, classification and prognosis of tumors, line PC-3M-1E8 (1E8-H) and the low metastatic line PC-3M- as well as for pinpointing molecular targets for improving 2B4 (2B4-L) were used for comparative proteomic analysis by treatment outcomes (2). two-dimensional gel electrophoresis, followed by matrix-assisted It is known that tumor progression to malignancy requires laser desorption/time of flight mass spectrometry (MALDI- a change from an epithelial phenotype to a fibroblast or TOF-MS). -

The Activator Protein-1 Transcription Factor in Respiratory Epithelium Carcinogenesis
Subject Review The Activator Protein-1 Transcription Factor in Respiratory Epithelium Carcinogenesis Michalis V. Karamouzis,1 Panagiotis A. Konstantinopoulos,1,2 and Athanasios G. Papavassiliou1 1Department of Biological Chemistry, Medical School, University of Athens, Athens, Greece and 2Division of Hematology-Oncology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts Abstract Much of the current anticancer research effort is focused on Respiratory epithelium cancers are the leading cause cell-surface receptors and their cognate upstream molecules of cancer-related death worldwide. The multistep natural because they provide the easiest route for drugs to affect history of carcinogenesis can be considered as a cellular behavior, whereas agents acting at the level of gradual accumulation of genetic and epigenetic transcription need to invade the nucleus. However, the aberrations, resulting in the deregulation of cellular therapeutic effect of surface receptor manipulation might be homeostasis. Growing evidence suggests that cross- considered less than specific because their actions are talk between membrane and nuclear receptor signaling modulated by complex interacting downstream signal trans- pathways along with the activator protein-1 (AP-1) duction pathways. A pivotal transcription factor during cascade and its cofactor network represent a pivotal respiratory epithelium carcinogenesis is activator protein-1 molecular circuitry participating directly or indirectly in (AP-1). AP-1–regulated genes include important modulators of respiratory epithelium carcinogenesis. The crucial role invasion and metastasis, proliferation, differentiation, and of AP-1 transcription factor renders it an appealing survival as well as genes associated with hypoxia and target of future nuclear-directed anticancer therapeutic angiogenesis (7). Nuclear-directed therapeutic strategies might and chemoprevention approaches. -

Daam2 Couples Translocation and Clustering of Wnt Receptor Signalosomes Through Rac1 Carlo D
© 2021. Published by The Company of Biologists Ltd | Journal of Cell Science (2021) 134, jcs251140. doi:10.1242/jcs.251140 RESEARCH ARTICLE Daam2 couples translocation and clustering of Wnt receptor signalosomes through Rac1 Carlo D. Cristobal1,QiYe2, Juyeon Jo2, Xiaoyun Ding3, Chih-Yen Wang2, Diego Cortes2, Zheng Chen4 and Hyun Kyoung Lee1,3,5,* ABSTRACT Dynamic polymerization of the Dishevelled proteins functions at Wnt signaling plays a critical role in development across species and the core of the Wnt signalosome by interacting with both the is dysregulated in a host of human diseases. A key step in signal Frizzled Wnt receptors and low-density lipoprotein receptor-related transduction is the formation of Wnt receptor signalosomes, during protein 5/6 (LRP5/6), leading to recruitment of Axin proteins β which a large number of components translocate to the membrane, from the -catenin destruction complex (MacDonald et al., 2009; cluster together and amplify downstream signaling. However, the Schwarz-Romond et al., 2007). However, the exact composition molecular processes that coordinate these events remain poorly and mechanisms of signalosome assembly at the plasma membrane defined. Here, we show that Daam2 regulates canonical Wnt remain unclear. signaling via the PIP –PIP5K axis through its association with Rac1. The hallmark of canonical Wnt signaling is the accumulation and 2 β Clustering of Daam2-mediated Wnt receptor complexes requires both translocation of -catenin into the nucleus for gene transcription, β Rac1 and PIP5K, and PIP5K promotes membrane localization of these whereas non-canonical Wnt signaling is -catenin independent and complexes in a Rac1-dependent manner. Importantly, the localization involves assembly/disassembly of the actin cytoskeleton, polarized of Daam2 complexes and Daam2-mediated canonical Wnt signaling is cell shape changes and cell migration (Niehrs, 2012; Schlessinger dependent upon actin polymerization. -

Plakoglobin Is Required for Effective Intermediate Filament Anchorage to Desmosomes Devrim Acehan1, Christopher Petzold1, Iwona Gumper2, David D
ORIGINAL ARTICLE Plakoglobin Is Required for Effective Intermediate Filament Anchorage to Desmosomes Devrim Acehan1, Christopher Petzold1, Iwona Gumper2, David D. Sabatini2, Eliane J. Mu¨ller3, Pamela Cowin2,4 and David L. Stokes1,2,5 Desmosomes are adhesive junctions that provide mechanical coupling between cells. Plakoglobin (PG) is a major component of the intracellular plaque that serves to connect transmembrane elements to the cytoskeleton. We have used electron tomography and immunolabeling to investigate the consequences of PG knockout on the molecular architecture of the intracellular plaque in cultured keratinocytes. Although knockout keratinocytes form substantial numbers of desmosome-like junctions and have a relatively normal intercellular distribution of desmosomal cadherins, their cytoplasmic plaques are sparse and anchoring of intermediate filaments is defective. In the knockout, b-catenin appears to substitute for PG in the clustering of cadherins, but is unable to recruit normal levels of plakophilin-1 and desmoplakin to the plaque. By comparing tomograms of wild type and knockout desmosomes, we have assigned particular densities to desmoplakin and described their interaction with intermediate filaments. Desmoplakin molecules are more extended in wild type than knockout desmosomes, as if intermediate filament connections produced tension within the plaque. On the basis of our observations, we propose a particular assembly sequence, beginning with cadherin clustering within the plasma membrane, followed by recruitment of plakophilin and desmoplakin to the plaque, and ending with anchoring of intermediate filaments, which represents the key to adhesive strength. Journal of Investigative Dermatology (2008) 128, 2665–2675; doi:10.1038/jid.2008.141; published online 22 May 2008 INTRODUCTION dense plaque that is further from the membrane and that Desmosomes are large macromolecular complexes that mediates the binding of intermediate filaments. -
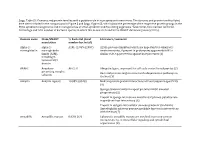
Supp. Table S2: Domains and Protein Families with a Putative Role in Host-Symbiont Interactions
Supp. Table S2: Domains and protein families with a putative role in host-symbiont interactions. The domains and protein families listed here were included in the comparisons in Figure 5 and Supp. Figure S5, which show the percentage of the respective protein groups in the Riftia symbiont metagenome and in metagenomes of other symbiotic and free-living organisms. % bacterial, total number bacterial: Percentage and total number of bacterial species in which this domain is found in the SMART database (January 2019). Domain name Pfam/SMART % bacterial (total Literature/comment annotation number bacterial) Alpha-2- alpha-2- A2M: 42.05% (2057) A2Ms: protease inhibitors which are important for eukaryotic macroglobulin macroglobulin innate immunity, if present in prokaryotes apparently fulfill a family (A2M), similar role, e.g. protection against host proteases (1) including N- terminal MG1 domain ANAPC Anaphase- APC2: 0 Ubiquitin ligase, important for cell cycle control in eukaryotes (2) promoting complex Bacterial proteins might interact with ubiquitination pathways in subunits the host (3) Ankyrin Ankyrin repeats 10.88% (8348) Mediate protein-protein interactions without sequence specificity (4) Sponge symbiont ankyrin-repeat proteins inhibit amoebal phagocytosis (5) Present in sponge microbiome metatranscriptomes, putative role in symbiont-host interactions (6) Present in obligate intracellular amoeba symbiont Candidatus Amoebophilus asiaticus genome, probable function in interactions with the host (7) Armadillo Armadillo repeats 0.83% (67) -

99978 Fascin (55K-2) Mouse Mab (IHC Formulated)
Revision 1 C 0 2 - t Fascin (55K-2) Mouse mAb (IHC a e r o t Formulated) S Orders: 877-616-CELL (2355) [email protected] 8 Support: 877-678-TECH (8324) 7 9 Web: [email protected] 9 www.cellsignal.com 9 # 3 Trask Lane Danvers Massachusetts 01923 USA For Research Use Only. Not For Use In Diagnostic Procedures. Applications: Reactivity: Sensitivity: MW (kDa): Source/Isotype: UniProt ID: Entrez-Gene Id: IHC-P H M Endogenous 55 Mouse IgG1 Q16658 6624 Product Usage Information Application Dilution Immunohistochemistry (Paraffin) 1:100 Storage Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA, 50% glycerol and less than 0.02% sodium azide. Store at –20°C. Do not aliquot the antibody. Specificity / Sensitivity Fascin (55K-2) Mouse mAb (IHC Formulated) recognizes endogenous levels of total fascin protein. Species Reactivity: Human, Mouse Source / Purification Monoclonal antibody is produced by immunizing animals with fascin protein purified from HeLa cells. Background Fascin is a monomeric, globular protein that plays a central role in regulating the structure and function of the cortical actin cytoskeleton (1). Fascin promotes cross-linkage of parallel actin filaments during the formation of cell protrusions (lamellipodia and filopodia), and therefore plays an important role in regulating cell migration (2). It has been reported that fascin may also regulate filopodia formation by a mechanism independent of its actin- bundling functions (3), though less is known about this mechanism of action. Research studies have shown that increased fascin expression is associated with increased motility and invasiveness of neoplastic cells, including breast, colon, prostate, and esophageal squamous cell carcinomas (4-6). -

Fascin Is Regulated by Slug, Promotes Progression of Pancreatic Cancer in Mice, and Is Associated with Patient Outcomes Ang Li,1 Jennifer P
Gastroenterology 2014;146:1386–1396 BASIC AND TRANSLATIONAL—PANCREAS Fascin Is Regulated by Slug, Promotes Progression of Pancreatic Cancer in Mice, and Is Associated With Patient Outcomes Ang Li,1 Jennifer P. Morton,1 YaFeng Ma,1 Saadia A. Karim,1 Yan Zhou,1 William J. Faller,1 Emma F. Woodham,1 Hayley T. Morris,1 Richard P. Stevenson,1 Amelie Juin,1 Nigel B. Jamieson,2 Colin J. MacKay,2 C. Ross Carter,2 Hing Y. Leung,1 Shigeko Yamashiro,3 Karen Blyth,1 Owen J. Sansom,1 and Laura M. Machesky1 1CRUK Beatson Institute for Cancer Research, College of Medical Veterinary and Life Sciences, University of Glasgow, Glasgow, UK; 2Department of Surgery, West of Scotland Pancreatic Unit, Glasgow Royal Infirmary, Glasgow, UK; and 3Department of Molecular Biology and Biochemistry, Rutgers University, Piscataway, New Jersey BACKGROUND & AIMS: Pancreatic ductal adenocarcinoma who undergo resection for localized lesions develop recur- (PDAC) is often lethal because it is highly invasive and metas- rent or metastatic disease.2 Consequently, the development tasizes rapidly. The actin-bundling protein fascin has been of more effective strategies to combat metastasis is of identified as a biomarker of invasive and advanced PDAC and paramount importance. regulates cell migration and invasion in vitro. We investigated Human PDAC arises from pancreatic intraepithelial fascin expression and its role in PDAC progression in mice. neoplasias (PanINs) frequently driven by activating muta- METHODS: G12D R172H We used KRas p53 Pdx1-Cre (KPC) mice tions in KRas,3 followed by loss or mutation of tumor sup- fi to investigate the effects of fascin de ciency on development of pressors, such as p53. -

Signal Transduction and the Ets Family of Transcription Factors
Oncogene (2000) 19, 6503 ± 6513 ã 2000 Macmillan Publishers Ltd All rights reserved 0950 ± 9232/00 $15.00 www.nature.com/onc Signal transduction and the Ets family of transcription factors John S Yordy1 and Robin C Muise-Helmericks*,1,2 1Center for Molecular and Structural Biology, Hollings Cancer Center, Medical University of South Carolina, Charleston, South Carolina, SC 29403, USA; 2Department of Cell Biology and Anatomy, Medical University of South Carolina, Charleston, South Carolina, SC 29403, USA Cellular responses to environmental stimuli are con- expression required for cellular growth, dierentiation trolled by a series of signaling cascades that transduce and survival. One group of downstream eectors of extracellular signals from ligand-activated cell surface these signaling pathways is the Ets family of transcrip- receptors to the nucleus. Although most pathways were tion factors. Ets family members can also be initially thought to be linear, it has become apparent that considered upstream eectors of signal transduction there is a dynamic interplay between signaling pathways pathways controlling the expression of a number of that result in the complex pattern of cell-type speci®c signaling components including both receptor tyrosine responses required for proliferation, dierentiation and kinases and intermediate signaling molecules. survival. One group of nuclear eectors of these The Ets family of transcription factors is de®ned by signaling pathways are the Ets family of transcription a conserved winged helix ± turn ± helix DNA binding factors, directing cytoplasmic signals to the control of domain (Papas et al., 1989; Wasylyk et al., 1993; gene expression. This family is de®ned by a highly Werner et al., 1995). -

Null Mutation of the Fascin2 Gene by TALEN Leading to Progressive Hearing Loss and Retinal Degeneration in C57BL/6J Mice
G3: Genes|Genomes|Genetics Early Online, published on August 6, 2018 as doi:10.1534/g3.118.200405 Null mutation of the Fascin2 gene by TALEN leading to progressive hearing loss and retinal degeneration in C57BL/6J mice Xiang Liu1,3,§, Mengmeng Zhao1,2,§, Yi Xie1,2, §, Ping Li1, Oumei Wang1 , Bingxin Zhou1,2, Linlin Yang1,4, Yao Nie1, Lin Cheng1, Xicheng Song1,5, Changzhu Jin1,3,**, Fengchan Han1,2,* 1Key Laboratory for Genetic Hearing Disorders in Shandong, Binzhou Medical University, 346 Guanhai Road, Yantai264003, Shandong, P. R. China 2Department of Biochemistry and Molecular Biology, Binzhou Medical University, 346 Guanhai Road, Yantai264003, Shandong, P. R. China 3Department of Human Anatomy and Histology and Embryology, Binzhou Medical University, 346 Guanhai Road, Yantai264003, Shandong, P. R. China 4Department of Otorhinolaryngology-Head and Neck Surgery, Yuhuangding Hospital, 20 East Yuhuangding Road, Yantai 264000, Shandong, P.R .China § These authors contribute equally *The First Corresponding Author: [email protected] **The Second Corresponding Author: [email protected] Key Laboratory for Genetic Hearing Disorders in Shandong, Department of Biochemistry and Molecular Biology, Binzhou Medical University, 346 Guanhai Road, Yantai264003, Shandong, P. R. China © The Author(s) 2013. Published by the Genetics Society of America. Abstract Fascin2 (FSCN2) is an actin cross-linking protein that is mainly localized in retinas and in the stereocilia of hair cells. Earlier studies showed that a deletion mutation in human FASCIN2 (FSCN2) gene could cause autosomal dominant retinitis pigmentosa. Recent studies have indicated that a missense mutation in mouse Fscn2 gene (R109H) can contribute to the early onset of hearing loss in DBA/2J mice.