Neuroimage 231 (2021) 117850
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Name of Recognized Medical Schools (Foreign)
1 Name of Recognized Medical Schools (Foreign) Expired AUSTRALIA 1 School of Medicine, Faculty of Heath, University of Tasmania, Tasmania, Australia (5 years Program) 9 Jan Main Affiliated Hospitals 2021 1. Royal H obart Hospital 2. Launceston Gen Hospital 3. NWest Region Hospital 2 Melbourne Medical School, University of Melbourne, Victoria, Australia (4 years Program) 1 Mar Main Affiliated Hospitals 2022 1. St. Vincent’s Public Hospital 2. Epworth Hospital Richmond 3. Austin Health Hospital 4. Bendigo Hospital 5. Western Health (Sunshine, Footscray & Williamstown) 6. Royal Melbourne Hospital Affiliated Hospitals 1. Pater MacCallum Cancer Centre 2. Epworth Hospital Freemasons 3. The Royal Women’s Hospital 4. Mercy Hospital for Women 5. The Northern Hospital 6. Goulburn Valley Health 7. Northeast Health 8. Royal Children’s Hospital 3 School of Medicine and Public Health, University of Newcastle, New South Wales, Australia (5 years Program) 3 May Main Affiliated Hospitals 2022 1.Gosford School 2. John Hunter Hospital Affiliated Hospitals 1. Wyong Hospital 2. Calvary Mater Hospital 3. Belmont Hospital 4. Maitland Hospital 5. Manning Base Hospital & University of Newcastle Department of Rural Health 6. Tamworth Hospital 7. Armidale Hospital 4 Faculty of Medicine, Nursing and Health Sciences, Monash University, Australia (4 and 5 years Program) 8 Nov Main Affiliated Hospitals 1. Eastern Health Clinical School: EHCS 5 Hospitals 2022 2. Southern School for Clinical Sciences: SCS 5 Hospitals 3. Central Clinical School จ ำนวน 6 Hospitals 4. School of Rural Health จ ำนวน 7 Hospital 5 Sydney School of Medicine (Sydney Medical School), Faculty of Medicine and Health, University of Sydney, Australia 12 Dec (4 years Program) 2023 2 Main Affiliated Hospitals 1. -
Curriculum Vitae
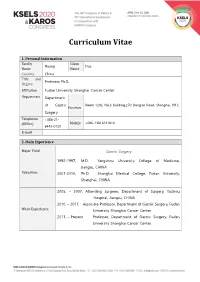
Curriculum Vitae 1. Personal Information Family Given Huang Hua Name Name Country China Title and Professor, Ph.D. Degree Affiliation Fudan University Shanghai Cancer Center Department Department of Gastric Room 1203, No.3 Building,270 Dong’an Road, Shanghai, P.R.C. Position Surgery Telephone +086-21- (Office) Mobile +086-13816151816 6443-0130 E-mail 2. Main Experience Major Field Gastric Surgery 1992-1997, M.D. Yangzhou University College of Medicine, Jiangsu, CHINA Education 2007-2010, Ph.D. Shanghai Medical College, Fudan University, Shanghai, CHINA 2005, – 2007, Attending surgeon, Department of Surgery, Taizhou Hospital, Jiangsu, CHINA 2010, – 2017, Associate Professor, Department of Gastric Surgery, Fudan Work Experience University Shanghai Cancer Center. 2017, – Present Professor, Department of Gastric Surgery, Fudan University Shanghai Cancer Center. 1. Hua Huang, XiaoFei Zhang, HaiJun Zhou, YuHua Xue, QiongZhu Dong, QingHai Ye, LunXiu Qin. Expression of osteopontin and caspase-3 and its prognostic significance in hepatocellular carcinoma patients after curative resection. Cancer Science. 2010;101(5):1314-1319. 2. HaiJun Zhou, Hua Huang, Jiong Shi, Yue Zhao, QiongZhu Dong, HuLiang Jia,YinKun Liu, QingHai Ye,huichuan sun, Xiaodong zhu, LiYun Fu, Kun Guo, DongMei Gao, Jian Sun, ZhaoWei Yan, Ning Ren, Zhaoyou Tang, LunXiu Qin. The Prognostic Value of IL-2 and IL-15 in Peritumoral Hepatic Tissues for Hepatitis B-related Hepatocellular Carcinoma Patients after Curative Resection. Gut. 2010 Dec;59(12):1699-708. (co-first author). 3. Hua Huang, Wei Wang, Zhong Chen, Jie-Jie Jin, Zi-Wen Long, Hong Cai, Xiao-Wen Liu, Ye Zhou and Ya-Nong Wang. Prognostic factors and survival Research Interests in patients with gastric stump cancer. -

20180115-CHN-Eng.Pdf (1.114Mb)
Meeting Report GO WHO CHINA: WORKING IN WHO 15 January 2018 Beijing, People's Republic of China GO WHO China: Working in WHO 15 January 2018 Beijing, People's Republic of China WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC English only MEETING REPORT GO WHO CHINA: WORKING IN WHO Convened by: WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC Beijing, People’s Republic of China 15 January 2018 Not for sale Printed and distributed by: World Health Organization Regional Office for the Western Pacific Manila, Philippines February 2018 NOTE The views expressed in this report are those of the participants of the Go WHO Workshops to Improve Geographical Representation of WHO Staff and do not necessarily reflect the policies of the conveners. This report has been prepared by the World Health Organization Regional Office for the Western Pacific for Member States in the Region and for those who participated in the Go WHO Workshops to Improve Geographical Representation of WHO Staff in Beijing, People’s Republic of China on 15 January 2018. CONTENTS SUMMARY ............................................................................................................................................ 1 1. INTRODUCTION .............................................................................................................................. 2 1.1 Background ................................................................................................................................... 2 1.2 Workshop objectives .................................................................................................................... -

Prof. Weiliu Qiu: Be a “Bowing” Doctor
Meet the Professor Page 1 of 9 Prof. Weiliu Qiu: be a “bowing” doctor Received: 13 July 2019; Accepted: 01 August 2019; Published: 09 August 2019. doi: 10.21037/fomm.2019.08.02 View this article at: http://dx.doi.org/10.21037/fomm.2019.08.02 Upon seeing his “savior” in the ward, the old man would get so excited that he would get up from bed outright. “Slowly, slowly!” academician Weiliu Qiu hurriedly held up the old man, bent down and picked up the slippers on the ground to put on his feet. The old man was completely bewildered at the sight of a 70-year-old academician putting on shoes for him. “How can this be done? How can this be done?” This small moment happened more than a decade ago, but is still heartwarming to this day. Weiliu Qiu (Figure 1) is one of the founders and pioneers of oral and maxillofacial surgery in China, and the first member of the Chinese Academy of Engineering in the field of stomatology. He was the former president of Shanghai Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University and director of the university’s College Figure 1 Academician Weiliu Qiu. of Stomatology. During his 60-year career, he has set a number of records in the field of oral and maxillofacial surgery, and cured numerous patients. surgery, and oral and maxillofacial repair/reconstruction When reflecting on his life, Prof. Qiu says in an surgery in China. He has won three National Invention emotional tone, “patients are teachers of doctors, and they bring Awards and Science and Technology Progress Awards, and us more than what we have given to them. -

Chinese Guidelines for the Diagnosis and Treatment of Hospital- Acquired Pneumonia and Ventilator-Associated Pneumonia in Adults (2018 Edition)
2616 Guideline Chinese guidelines for the diagnosis and treatment of hospital- acquired pneumonia and ventilator-associated pneumonia in adults (2018 Edition) Yi Shi1#, Yi Huang2#, Tian-Tuo Zhang3#, Bin Cao4#, Hui Wang5#, Chao Zhuo6#, Feng Ye6#, Xin Su1#, Hong Fan7#, Jin-Fu Xu8#, Jing Zhang9#, Guo-Xiang Lai10#, Dan-Yang She11#, Xiang-Yan Zhang12, Bei He13, Li-Xian He9, You-Ning Liu14, Jie-Ming Qu15; on behalf of Infection Study Group of Chinese Thoracic Society, Chinese Medical Association 1Department of Pulmonary and Critical Care Medicine, Nanjing Jinling Hospital, Nanjing University, School of Medicine, Nanjing 210002, China; 2Department of Pulmonary and Critical Care Medicine, Shanghai Changhai hospital, Navy Medical University, Shanghai 200433, China; 3Department of Pulmonary and Critical Care Medicine, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630, China; 4Department of Pulmonary and Critical Care Medicine, China-Japan Friendship Hospital, Capital Medical University, Beijing 100029, China; 5Department of Clinical Laboratory Medicine, Peking University People’s Hospital, Beijing 100044, China; 6State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou 510120, China; 7Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu 610041, China; 8Department of Pulmonary and Critical -

The 2 Asia Medical Week
SYNERGIZED INNOVATION & SUSTAINED EVOLUTION THE 2nd ASIA MEDICAL WEEK ASIAN MEDICAL INNOVATION & DEVELOPMENT FORUM 2019 Fudan Zhongshan International Oncology Summit & Annual Conference of Fudan Zhongshan Cancer Center Shanghai / Shenzhen Oct.19-21 2019 ACTIVITIES RECAP 2018 South Korea 2019 Statistics 11 4 4 1000+ 30 81% Sub-Forums Private Sessions Clinial Exchange Visit Attendees Countries & Regions Top Management Presidents, Vice-Presidents, CEOs, Directors or Officers from Governments, MOHs & Hospitals. Overseas Visitors Armenia Australia Bangladesh Belarus Brazil China Egypt France Greece India Indonesia Italy Japan Kazakhstan Kenya Malaysia Myanmar Northern Cyprus Pakistan Philippines Russia Singapore South Korea Sri Lanka Thailand Turkey Ukraine UK US Vietnam Launching Ceremony Dear Professors, We take a great pleasure in inviting you to the 2019 Asian Medical Week, which is held in Shanghai from October 19th to October 21st , 2019. Asia Medical Week is a prominent academic event for promoting medical communication and cooperation in the Asia-Pacific region. With the theme focusing on "Medical Innovation & Development", 2019 Asia Medical Week is dedicated to providing an effective communication platform for international healthcare community and industry to showcase China’s latest oncology, promote sharing for high-quality healthcare resources and advocate high-tech cooperation for modern medicine. Towards a vision of "Collaborative Innovation, Sustainable Progress", Fudan University Zhongshan Hospital and Fudan University Zhong Shan Hospital Cancer Center (FUZSCC) will host the 2019 Asian Medical Week - - Medical Innovation & Development Forum, featuring 2019 Fudan Zhongshan International Oncology Summit, in concurrent with the annual meeting of FUZSCC. To pace the internationalization and development of China healthcare system through in-depth communication and collaboration between Asia-Pacific countries, the program will focus on the newest advances in tumor management and establish “the Belt and Road” Oncology Alliance during the event. -

Author Correction: KRAB-Type Zinc-Finger Proteins PITA and PISA
www.nature.com/cr www.cell-research.com CORRECTION Author Correction: KRAB-type zinc-finger proteins PITA and PISA specifically regulate p53-dependent glycolysis and mitochondrial respiration Shan Wang1,2,3, Zhiqiang Peng1,2, Siying Wang4, Lihua Yang4, Yuhan Chen1,2, Xue Kong4, Shanshan Song1,2, Pei Pei3, Chunyan Tian1,2, Hui Yan5, Peipei Ding6, Weiguo Hu6, Cui Hua Liu 7, Xin Zhang8, Fuchu He1,2 and Lingqiang Zhang1,2,9 Cell Research (2018) 28:605; https://doi.org/10.1038/s41422-018-0026-6 Correction to: Cell Research (2018) 0:1-21. https://doi.org/10.1038/ s41422-018-0008-8; published online 21 February 2018. We apologize for an error that we just found in the paper published online on 21 February 2018. The 1st row/1st column of Fig. 8g (PITA Normal) was inadvertently duplicated from 2nd row/ 1st column of Fig. 8i (PISA Adjacent colonic mucosa). The 2nd row/ 1st column of Fig. 8g (PISA Normal) was inadvertently duplicated from 2nd row/2nd column of Fig. 8g (PISA Distal colonic mucosa). A corrected version of Fig. 8g is provided below. No conclusion was affected by this error, but we apologize for not detecting it Fig. 8 PITA and PISA promote tumorigenesis. g Representative before publication and any inconvenience caused. images of immunohistochemical staining for PITA and PISA are shown. Scale bar, 50 μm 1State Key Laboratory of Proteomics, Beijing Proteome Research Center, National Center of Protein Sciences (Beijing), Beijing Institute of Lifeomics, Beijing, China; 2Department of Genomics and Proteomics, Beijing Institute of Radiation -

Differences in Treatment Effect Size Between Progression-Free Survival
2572 Original Article Differences in treatment effect size between progression-free survival and overall survival in anti-PD-1/PD-L1 inhibitors-based trials in advanced NSCLC: a systematic review and meta-analysis Zhirui Zhou1#, Shengxiang Ren2#, Lingxiao Chen3, Caicun Zhou2, Tao Jiang2,4 1Radiation Oncology Center, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, China; 2Department of Medical Oncology, Shanghai Pulmonary Hospital & Thoracic Cancer Institute, Tongji University School of Medicine, Shanghai, China; 3Institute of Bone and Joint Research, Kolling Institute, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, New South Wales, Australia; 4Department of Pulmonary Medicine, Shanghai Respiratory Research Institute, Zhongshan Hospital Affiliated to Fudan University, Shanghai, China Contributions: (I) Conception and design: T Jiang, S Ren, C Zhou; (II) Administrative support: T Jiang, S Ren, C Zhou; (III) Provision of study materials or patients: T Jiang, S Ren, C Zhou; (IV) Collection and assembly of data: T Jiang, Z Zhou, L Chen; (V) Data analysis and interpretation: T Jiang, Z Zhou, L Chen; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. #These authors contributed equally to this work. Correspondence to: Tao Jiang, PhD. Department of Medical Oncology, Shanghai Pulmonary Hospital & Thoracic Cancer Institute, Tongji University School of Medicine, No. 507, Zheng Min Road, Shanghai 200433, China; Department of Pulmonary Medicine, Shanghai Respiratory Research Institute, Zhongshan Hospital Affiliated to Fudan University, No. 180, Fenglin Road, Shanghai 200032, China. Email: [email protected]. Background: To investigate the differences in treatment effect sizes between progression-free survival (PFS) and overall survival (OS) in advanced non-small cell lung cancer (NSCLC) treated with programmed cell death 1 (PD-1) and its ligand (PD-L1) blockade-based treatments. -

Enhanced Activities of Blood Thiamine Diphosphatase and Monophosphatase in Alzheimer’S Disease
RESEARCH ARTICLE Enhanced Activities of Blood Thiamine Diphosphatase and Monophosphatase in Alzheimer's Disease Xiaoli Pan1,2☯, Shaoming Sang1,2☯, Guoqiang Fei1, Lirong Jin1, Huimin Liu2, Zhiliang Wang3, Hui Wang3, Chunjiu Zhong1,2* 1 Department of Neurology, Zhongshan Hospital & Shanghai Medical College, Fudan University, Shanghai, China, 2 State Key Laboratory of Medical Neurobiology, Institutes of Brain Science & Collaborative Innovation Center for Brain Science, Fudan University, Shanghai, China, 3 Regional Health Service Center of a11111 Xujiahui, Xuhui District, Shanghai, China ☯ These authors contributed equally to this work. * [email protected] Abstract OPEN ACCESS Citation: Pan X, Sang S, Fei G, Jin L, Liu H, Wang Z, et al. (2017) Enhanced Activities of Blood Background Thiamine Diphosphatase and Monophosphatase in Thiamine metabolites and activities of thiamine-dependent enzymes are impaired in Alzhei- Alzheimer's Disease. PLoS ONE 12(1): e0167273. mer's disease (AD). doi:10.1371/journal.pone.0167273 Editor: Marie-Claude Potier, Institut du cerveau et de la moelle epiniere, FRANCE Objective Received: April 17, 2016 To clarify the mechanism for the reduction of thiamine diphosphate (TDP), an active form of Accepted: November 12, 2016 thiamine and critical coenzyme of glucose metabolism, in AD. Published: January 6, 2017 Copyright: © 2017 Pan et al. This is an open access Methods article distributed under the terms of the Creative Forty-five AD patients clinically diagnosed and 38 age- and gender-matched control sub- Commons Attribution License, which permits unrestricted use, distribution, and reproduction in jects without dementia were voluntarily recruited. The contents of blood TDP, thiamine any medium, provided the original author and monophosphate (TMP), and thiamine, as well as the activities of thiamine diphosphatase source are credited. -

A Hydrostatic Pressure-Driven Passive Micropump Enhanced with Siphon-Based Autofill Function
Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is © The Royal Society of Chemistry 2018 A hydrostatic pressure-driven passive micropump enhanced with siphon-based autofill function Xiaolin Wang‡abc, Da Zhao‡d, Duc T.T. Phane, Jingquan Liuabc, Xiang Chenabc, Bin Yangabc, Christopher C.W. Hughesde, Weijia Zhang*fg, Abraham P. Lee*dh a Department of Micro/Nano Electronics, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China b National Key Laboratory of Science and Technology on Micro/Nano Fabrication, Department of Micro/Nano Electronics, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China c Key Laboratory for Thin Film and Microfabrication Technology (Ministry of Education), Department of Micro/Nano Electronics, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China d Department of Biomedical Engineering, University of California, Irvine, CA 92697, USA. E-mail: [email protected]; Fax: +1 (949) 824 1727;Tel: +1 (949) 824 9691 e Department of Molecular Biology & Biochemistry, University of California, Irvine, CA 92697, USA f The Fifth People’s Hospital of Shanghai and Institutes of Biomedical Sciences, Shanghai Medical College, Fudan University, Shanghai 200032, China. E-mail: [email protected]; Tel: +86 (021) 5423 7385 g State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai 200433, China h Department of Mechanical and Aerospace Engineering, University of California, Irvine, CA 92697, USA ‡ These authors contributed equally to this work * These authors contributed equally as senior authors and correspondents to this work Corresponding authors: Weijia Zhang Email: [email protected]; Tel: +86 (021) 5423-7385. -

The Cost of Alzheimer's Disease in China and Re-Estimation of Costs Worldwide
Alzheimer’s & Dementia 14 (2018) 483-491 Featured Article The cost of Alzheimer’s disease in China and re-estimation of costs worldwide Jianping Jiaa,b,c,d,e,*, Cuibai Weia,*, Shuoqi Chena, Fangyu Lia, Yi Tanga, Wei Qina, Lina Zhaoa, Hongmei Jina, Hui Xua, Fen Wanga, Aihong Zhoua, Xiumei Zuoa, Liyong Wua, Ying Hana, Yue Hana, Liyuan Huanga, Qi Wanga, Dan Lia, Changbiao Chua, Lu Shia, Min Gonga, Yifeng Duf, Jiewen Zhangg, Junjian Zhangh, Chunkui Zhoui, Jihui Lvj, Yang Lvk, Haiqun Xiel, Yong Jim, Fang Lin, Enyan Yuo, Benyan Luop, Yanjiang Wangq, Shanshan Yangr, Qiumin Qus, Qihao Guot, Furu Liangu, Jintao Zhangv, Lan Tanw, Lu Shenx, Kunnan Zhangy, Jinbiao Zhangz, Dantao Pengaa, Muni Tangab, Peiyuan Lvac, Boyan Fangad, Lan Chuae, Longfei Jiaaf, Serge Gauthierag,* aDepartment of Neurology, Xuan Wu Hospital, Capital Medical University, Beijing, China bBeijing Key Laboratory of Geriatric Cognitive Disorders, Beijing, China cCenter of Alzheimer’s Disease, Beijing Institute for Brain Disorders, Beijing, China dKey Laboratory of Neurodegenerative Diseases, Ministry of Education, Beijing, China eNational Clinical Research Center for Geriatric Disorders, Beijing, China fDepartment of Neurology, Shandong Provincial Hospital, Shandong University, Jinan, China gDepartment of Neurology, Henan Provincial People’s Hospital, Zhengzhou, China hDepartment of Neurology, Zhongnan Hospital of Wuhan University, Wuhan, China iDepartment of Neurology, The First Teaching Hospital of Jilin University, Changchun, China jDementia Unit, Beijing Geriatric Hospital, Beijing, -

A Nomogram to Predict the Overall Survival of Patients with Symptomatic Extensive-Stage Small Cell Lung Cancer Treated with Thoracic Radiotherapy
2171 Original Article A nomogram to predict the overall survival of patients with symptomatic extensive-stage small cell lung cancer treated with thoracic radiotherapy Xun Yuan1#, Zhiqin Zheng2,3#, Fangfang Liu1, Yuan Gao1, Wenhui Zhang4, Rossana Berardi5, Pranshu Mohindra6, Zhengfei Zhu2,7,8, Jie Lin4, Qian Chu1 1Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; 2Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China; 3Department of Radiation Oncology, Minhang Branch Hospital, Fudan University Shanghai Cancer Center, Shanghai, China; 4Department of Oncology, The Second Affiliated Hospital of Kunming Medical University, Kunming, China; 5Clinica Oncologica, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria Ospedali Riuniti Umberto I, GM Lancisi, G Salesi di Ancona, Italy; 6Department of Radiation Oncology, University of Maryland School of Medicine, Baltimore, MD, USA; 7Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China; 8Institute of Thoracic Oncology, Fudan University, Shanghai, China Contributions: (I) Conception and design: X Yuan, Q Chu; (II) Administrative support: R Berardi, P Mohindra; (III) Provision of study materials or patients: Z Zheng, F Liu; (IV) Collection and assembly of data: Y Gao, W Zhang; (V) Data analysis and interpretation: Z Zhu, J Lin; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. #These authors contributed equally to this work. Correspondence to: Qian Chu, MD, PhD. Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, 1095 Jiefang Avenue, Wuhan 430030, China. Email: [email protected]; Jie Lin, MD. Department of Oncology, the Second Affiliated Hospital of Kunming Medical University, 374 Dianmian Avenue, Wuhua District, Kunming 650101, China.