Bioimage Informatics for Big Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
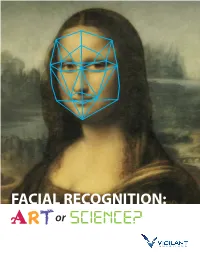
FACIAL RECOGNITION: a Or FACIALRT RECOGNITION: a Or RT Facial Recognition – Art Or Science?
FACIAL RECOGNITION: A or FACIALRT RECOGNITION: A or RT Facial Recognition – Art or Science? Both the public and law enforcement often misunderstand facial recognition technology. Hollywood would have you believe that with facial recognition technology, law enforcement can identify an individual from an image, while also accessing all of their personal information. The “Big Brother” narrative makes good television, but it vastly misrepresents how law enforcement agencies actually use facial recognition. Additionally, crime dramas like “CSI,” and its endless spinoffs, spin the narrative that law enforcement easily uses facial recognition Roger Rodriguez joined to generate matches. In reality, the facial recognition process Vigilant Solutions after requires a great deal of manual, human analysis and an image of serving over 20 years a certain quality to make a possible match. To date, the quality with the NYPD, where he threshold for images has been hard to reach and has often spearheaded the NYPD’s first frustrated law enforcement looking to generate effective leads. dedicated facial recognition unit. The unit has conducted Think of facial recognition as the 21st-century evolution of the more than 8,500 facial sketch artist. It holds the promise to be much more accurate, recognition investigations, but in the end it is still just the source of a lead that needs to be with over 3,000 possible matches and approximately verified and followed up by intelligent police work. 2,000 arrests. Roger’s enhancement techniques are Today, innovative facial now recognized worldwide recognition technology and have changed law techniques make it possible to enforcement’s approach generate investigative leads to the utilization of facial regardless of image quality. -

Bioimage Analysis Tools
Bioimage Analysis Tools Kota Miura, Sébastien Tosi, Christoph Möhl, Chong Zhang, Perrine Paul-Gilloteaux, Ulrike Schulze, Simon Norrelykke, Christian Tischer, Thomas Pengo To cite this version: Kota Miura, Sébastien Tosi, Christoph Möhl, Chong Zhang, Perrine Paul-Gilloteaux, et al.. Bioimage Analysis Tools. Kota Miura. Bioimage Data Analysis, Wiley-VCH, 2016, 978-3-527-80092-6. hal- 02910986 HAL Id: hal-02910986 https://hal.archives-ouvertes.fr/hal-02910986 Submitted on 3 Aug 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 2 Bioimage Analysis Tools 1 2 3 4 5 6 Kota Miura, Sébastien Tosi, Christoph Möhl, Chong Zhang, Perrine Pau/-Gilloteaux, - Ulrike Schulze,7 Simon F. Nerrelykke,8 Christian Tischer,9 and Thomas Penqo'" 1 European Molecular Biology Laboratory, Meyerhofstraße 1, 69117 Heidelberg, Germany National Institute of Basic Biology, Okazaki, 444-8585, Japan 2/nstitute for Research in Biomedicine ORB Barcelona), Advanced Digital Microscopy, Parc Científic de Barcelona, dBaldiri Reixac 1 O, 08028 Barcelona, Spain 3German Center of Neurodegenerative -

Other Departments and Institutes Courses 1
Other Departments and Institutes Courses 1 Other Departments and Institutes Courses About Course Numbers: 02-250 Introduction to Computational Biology Each Carnegie Mellon course number begins with a two-digit prefix that Spring: 12 units designates the department offering the course (i.e., 76-xxx courses are This class provides a general introduction to computational tools for biology. offered by the Department of English). Although each department maintains The course is divided into two halves. The first half covers computational its own course numbering practices, typically, the first digit after the prefix molecular biology and genomics. It examines important sources of biological indicates the class level: xx-1xx courses are freshmen-level, xx-2xx courses data, how they are archived and made available to researchers, and what are sophomore level, etc. Depending on the department, xx-6xx courses computational tools are available to use them effectively in research. may be either undergraduate senior-level or graduate-level, and xx-7xx In the process, it covers basic concepts in statistics, mathematics, and courses and higher are graduate-level. Consult the Schedule of Classes computer science needed to effectively use these resources and understand (https://enr-apps.as.cmu.edu/open/SOC/SOCServlet/) each semester for their results. Specific topics covered include sequence data, searching course offerings and for any necessary pre-requisites or co-requisites. and alignment, structural data, genome sequencing, genome analysis, genetic variation, gene and protein expression, and biological networks and pathways. The second half covers computational cell biology, including biological modeling and image analysis. It includes homework requiring Computational Biology Courses modification of scripts to perform computational analyses. -

An Introduction to Image Analysis Using Imagej
An introduction to image analysis using ImageJ Mark Willett, Imaging and Microscopy Centre, Biological Sciences, University of Southampton. Pete Johnson, Biophotonics lab, Institute for Life Sciences University of Southampton. 1 “Raw Images, regardless of their aesthetics, are generally qualitative and therefore may have limited scientific use”. “We may need to apply quantitative methods to extrapolate meaningful information from images”. 2 Examples of statistics that can be extracted from image sets . Intensities (FRET, channel intensity ratios, target expression levels, phosphorylation etc). Object counts e.g. Number of cells or intracellular foci in an image. Branch counts and orientations in branching structures. Polarisations and directionality . Colocalisation of markers between channels that may be suggestive of structure or multiple target interactions. Object Clustering . Object Tracking in live imaging data. 3 Regardless of the image analysis software package or code that you use….. • ImageJ, Fiji, Matlab, Volocity and IMARIS apps. • Java and Python coding languages. ….image analysis comprises of a workflow of predefined functions which can be native, user programmed, downloaded as plugins or even used between apps. This is much like a flow diagram or computer code. 4 Here’s one example of an image analysis workflow: Apply ROI Choose Make Acquisition Processing to original measurement measurements image type(s) Thresholding Save to ROI manager Make binary mask Make ROI from binary using “Create selection” Calculate x̄, Repeat n Chart data SD, TTEST times and interpret and Δ 5 A few example Functions that can inserted into an image analysis workflow. You can mix and match them to achieve the analysis that you want. -

A Review on Image Segmentation Clustering Algorithms Devarshi Naik , Pinal Shah
Devarshi Naik et al, / (IJCSIT) International Journal of Computer Science and Information Technologies, Vol. 5 (3) , 2014, 3289 - 3293 A Review on Image Segmentation Clustering Algorithms Devarshi Naik , Pinal Shah Department of Information Technology, Charusat University CSPIT, Changa, di.Anand, GJ,India Abstract— Clustering attempts to discover the set of consequential groups where those within each group are more closely related to one another than the others assigned to different groups. Image segmentation is one of the most important precursors for image processing–based applications and has a crucial impact on the overall performance of the developed systems. Robust segmentation has been the subject of research for many years, but till now published work indicates that most of the developed image segmentation algorithms have been designed in conjunction with particular applications. The aim of the segmentation process consists of dividing the input image into several disjoint regions with similar characteristics such as colour and texture. Keywords— Clustering, K-means, Fuzzy C-means, Expectation Maximization, Self organizing map, Hierarchical, Graph Theoretic approach. Fig. 1 Similar data points grouped together into clusters I. INTRODUCTION II. SEGMENTATION ALGORITHMS Images are considered as one of the most important Image segmentation is the first step in image analysis medium of conveying information. The main idea of the and pattern recognition. It is a critical and essential image segmentation is to group pixels in homogeneous component of image analysis system, is one of the most regions and the usual approach to do this is by ‘common difficult tasks in image processing, and determines the feature. Features can be represented by the space of colour, quality of the final result of analysis. -

Image Analysis for Face Recognition
Image Analysis for Face Recognition Xiaoguang Lu Dept. of Computer Science & Engineering Michigan State University, East Lansing, MI, 48824 Email: [email protected] Abstract In recent years face recognition has received substantial attention from both research com- munities and the market, but still remained very challenging in real applications. A lot of face recognition algorithms, along with their modifications, have been developed during the past decades. A number of typical algorithms are presented, being categorized into appearance- based and model-based schemes. For appearance-based methods, three linear subspace analysis schemes are presented, and several non-linear manifold analysis approaches for face recognition are briefly described. The model-based approaches are introduced, including Elastic Bunch Graph matching, Active Appearance Model and 3D Morphable Model methods. A number of face databases available in the public domain and several published performance evaluation results are digested. Future research directions based on the current recognition results are pointed out. 1 Introduction In recent years face recognition has received substantial attention from researchers in biometrics, pattern recognition, and computer vision communities [1][2][3][4]. The machine learning and com- puter graphics communities are also increasingly involved in face recognition. This common interest among researchers working in diverse fields is motivated by our remarkable ability to recognize peo- ple and the fact that human activity is a primary concern both in everyday life and in cyberspace. Besides, there are a large number of commercial, security, and forensic applications requiring the use of face recognition technologies. These applications include automated crowd surveillance, ac- cess control, mugshot identification (e.g., for issuing driver licenses), face reconstruction, design of human computer interface (HCI), multimedia communication (e.g., generation of synthetic faces), 1 and content-based image database management. -

Analysis of Artificial Intelligence Based Image Classification Techniques Dr
Journal of Innovative Image Processing (JIIP) (2020) Vol.02/ No. 01 Pages: 44-54 https://www.irojournals.com/iroiip/ DOI: https://doi.org/10.36548/jiip.2020.1.005 Analysis of Artificial Intelligence based Image Classification Techniques Dr. Subarna Shakya Professor, Department of Electronics and Computer Engineering, Central Campus, Institute of Engineering, Pulchowk, Tribhuvan University. Email: [email protected]. Abstract: Time is an essential resource for everyone wants to save in their life. The development of technology inventions made this possible up to certain limit. Due to life style changes people are purchasing most of their needs on a single shop called super market. As the purchasing item numbers are huge, it consumes lot of time for billing process. The existing billing systems made with bar code reading were able to read the details of certain manufacturing items only. The vegetables and fruits are not coming with a bar code most of the time. Sometimes the seller has to weight the items for fixing barcode before the billing process or the biller has to type the item name manually for billing process. This makes the work double and consumes lot of time. The proposed artificial intelligence based image classification system identifies the vegetables and fruits by seeing through a camera for fast billing process. The proposed system is validated with its accuracy over the existing classifiers Support Vector Machine (SVM), K-Nearest Neighbor (KNN), Random Forest (RF) and Discriminant Analysis (DA). Keywords: Fruit image classification, Automatic billing system, Image classifiers, Computer vision recognition. 1. Introduction Artificial intelligences are given to a machine to work on its task independently without need of any manual guidance. -

Face Recognition on a Smart Image Sensor Using Local Gradients
sensors Article Face Recognition on a Smart Image Sensor Using Local Gradients Wladimir Valenzuela 1 , Javier E. Soto 1, Payman Zarkesh-Ha 2 and Miguel Figueroa 1,* 1 Department of Electrical Engineering, Universidad de Concepción, Concepción 4070386, Chile; [email protected] (W.V.); [email protected] (J.E.S.) 2 Department of Electrical and Computer Engineering (ECE), University of New Mexico, Albuquerque, NM 87131-1070, USA; [email protected] * Correspondence: miguel.fi[email protected] Abstract: In this paper, we present the architecture of a smart imaging sensor (SIS) for face recognition, based on a custom-design smart pixel capable of computing local spatial gradients in the analog domain, and a digital coprocessor that performs image classification. The SIS uses spatial gradients to compute a lightweight version of local binary patterns (LBP), which we term ringed LBP (RLBP). Our face recognition method, which is based on Ahonen’s algorithm, operates in three stages: (1) it extracts local image features using RLBP, (2) it computes a feature vector using RLBP histograms, (3) it projects the vector onto a subspace that maximizes class separation and classifies the image using a nearest neighbor criterion. We designed the smart pixel using the TSMC 0.35 µm mixed- signal CMOS process, and evaluated its performance using postlayout parasitic extraction. We also designed and implemented the digital coprocessor on a Xilinx XC7Z020 field-programmable gate array. The smart pixel achieves a fill factor of 34% on the 0.35 µm process and 76% on a 0.18 µm process with 32 µm × 32 µm pixels. The pixel array operates at up to 556 frames per second. -
Image Analysis Image Analysis
PHYS871 Clinical Imaging ApplicaBons Image Analysis Image Analysis The Basics The Basics Dr Steve Barre April 2016 PHYS871 Clinical Imaging Applicaons / Image Analysis — The Basics 1 IntroducBon Image Processing Kernel Filters Rank Filters Fourier Filters Image Analysis Fourier Techniques Par4cle Analysis Specialist Solu4ons PHYS871 Clinical Imaging Applicaons / Image Analysis — The Basics 2 Image Display An image is a 2-dimensional collec4on of pixel values that can be displayed or printed by assigning a shade of grey (or colour) to every pixel value. PHYS871 Clinical Imaging Applicaons / Image Analysis — The Basics / Image Display 3 Image Display An image is a 2-dimensional collec4on of pixel values that can be displayed or printed by assigning a shade of grey (or colour) to every pixel value. PHYS871 Clinical Imaging Applicaons / Image Analysis — The Basics / Image Display 4 Spaal Calibraon For image analysis to produce meaningful results, the spaal calibraon of the image must be known. If the data acquisi4on parameters can be read from the image (or parameter) file then the spaal calibraon of the image can be determined. For simplicity and clarity, spaal calibraon will not be indicated on subsequent images. PHYS871 Clinical Imaging Applicaons / Image Analysis — The Basics / Image Display / Calibraon 5 SPM Image Display With Scanning Probe Microscope images there is no guarantee that the sample surface is level (so that the z values of the image are, on average, the same across the image). Raw image data A[er compensang for 4lt PHYS871 Clinical Imaging Applicaons / Image Analysis — The Basics / SPM Image Display 6 SPM Image Display By treang each scan line of an SPM image independently, anomalous jumps in the apparent height of the image (produced, for example, in STMs by abrupt changes in the tunnelling condi4ons) can be corrected for. -

Deep Learning in Medical Image Analysis
Journal of Imaging Editorial Deep Learning in Medical Image Analysis Yudong Zhang 1,* , Juan Manuel Gorriz 2 and Zhengchao Dong 3 1 School of Informatics, University of Leicester, Leicester LE1 7RH, UK 2 Department of Signal Theory, Telematics and Communications, University of Granada, 18071 Granada, Spain; [email protected] 3 Molecular Imaging and Neuropathology Division, Columbia University and New York State Psychiatric Institute, New York, NY 10032, USA; [email protected] * Correspondence: [email protected] Over recent years, deep learning (DL) has established itself as a powerful tool across a broad spectrum of domains in imaging—e.g., classification, prediction, detection, seg- mentation, diagnosis, interpretation, reconstruction, etc. While deep neural networks were initially nurtured in the computer vision community, they have quickly spread over medical imaging applications. The accelerating power of DL in diagnosing diseases will empower physicians and speed-up decision making in clinical environments. Applications of modern medical instru- ments and digitalization of medical care have led to enormous amounts of medical images being generated in recent years. In the big data arena, new DL methods and computational models for efficient data processing, analysis, and modeling of the generated data are crucial for clinical applications and understanding the underlying biological process. The purpose of this Special Issue (SI) “Deep Learning in Medical Image Analysis” is to present and highlight novel algorithms, architectures, techniques, and applications of DL for medical image analysis. This SI called for papers in April 2020. It received more than 60 submissions from Citation: Zhang, Y.; Gorriz, J.M.; over 30 different countries. -

Applications Research on the Cluster Analysis in Image Segmentation Algorithm
2017 International Conference on Materials, Energy, Civil Engineering and Computer (MATECC 2017) Applications Research on the Cluster Analysis in Image Segmentation Algorithm Jiangyan Sun, Huiming Su, Yuan Zhou School of Engineering, Xi'an International University, Xi’an, 710077, China Keywords: Image segmentation algorithm, Cluster analysis, Fuzzy cluster. Abstract. Clustering analysis is an unsupervised classification method, which classifies the samples by classifying the similarity. In the absence of prior knowledge, the image segmentation can be done by cluster analysis. This paper gives the concept of clustering analysis and two common cluster algorithms, which are FCM and K-Means clustering. The applications of the two clustering algorithms in image segmentation are explored in the paper to provide some references for the relevant researchers. Introduction It is a structured analysis process for people to understand the image. People should decompose the image into organized regions with specific properties constitutes before the next step. To identify and analyze the target, it is necessary to separate them based on the process. It is possible to further deal with the target. Image segmentation is the process of segmenting an image into distinct regions and extracting regions of interest. The segmented regions have consistent attributes, such as grayscale, color and texture. Image segmentation plays an important role in image engineering, and it is the key step from image processing to image analysis. It relates to the target feature measurement and determines the target location; at the same time, the object hierarchy and extracting parameters of the target expression, feature measurement helps establish the image based on the structure of knowledge is an important basis for image understanding. -

AI in Image Analytics
International Journal of Computational Intelligence Research ISSN 0973-1873 Volume 15, Number 2 (2019), pp. 81-95 © Research India Publications http://www.ripublication.com AI In Image Analytics Sathya Narayanan PSV Data Analytics and AI - Topcoder Executive Summary The approach of "image analysis" in creating "artificial intelligence models" has created a buzz in the world of technology. "Image analysis" has enabled a greater understanding of the concerned process. The various aspects of image analysis have been discussed in detail, such as "analogue image processing", “digital image processing”, “and image pattern recognition”and“image acquisition”. The study has been done to explore the techniques used to develop “artificial intelligence”. The advantages and disadvantages of these models have been discussed which will enable the young researchers to understand which model will be appropriate to be used in the development of "artificial intelligence models". The image analysis with the consistent factor of face recognition will enable the readers to understand the efficiency of the system and cater to the areas where improvisation is needed. The approach has been implemented to understand the working of the ongoing process. The critical aspect of the mentioned topic has been discussed in detail. The study is intended to give the readers a detailed understanding of the problem and open new arenas for further research. 1. INTRODUCTION “Image analysis” could be quick consists of the factors which generate meaningful information concerning any objectifying picture form the digital media.“Artificial Intelligence Model (AI Model)” is the unique based reasoning system for increasing the usability of any concerning system in the digital world.