Paysandisia Archon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Syagrus Romanzoffiana [Cham.] Glassman](https://docslib.b-cdn.net/cover/5715/syagrus-romanzoffiana-cham-glassman-115715.webp)
Syagrus Romanzoffiana [Cham.] Glassman
SCIENTIFIC note Doi: https://doi.org/10.17584/rcch.2019v13i3.8363 Pre-depulping and depulping treatments and the emergence of queen palm seeds (Syagrus romanzoffiana [Cham.] Glassman) Tratamiento de pre-despulpado y despulpado sobre la emergencia de semillas de palma reina (Syagrus romanzoffiana [Cham.] Glassman) LUCAS MARQUEZAN NASCIMENTO1 EDUARDO PRADI VENDRUSCOLO2, 4 LUIZ FERNANDES CARDOSO CAMPOS1 LISMAÍRA GONÇALVES CAIXETA GARCIA1 LARISSA LEANDRO PIRES1 ALEXANDER SELEGUINI3 Syagrus romanzoffiana under conditions of Brazilian Cerrado. Photo: L.M. Nascimento ABSTRACT The propagation of the palm Syagrus romanzoffiano is done sexually with seeds, making the process of obtai- ning new plants slow and difficult, especially on large scales. In addition, seed germination is slow, uneven and susceptible to degradation and loss of vigor because of embryo deterioration, even under laboratory conditions. As a result of the lack of information on efficient depulping methods for queen palm fruits, the present study aimed to establish a depulping methodology that is less aggressive to embryos, maintaining emergence quality. This experiment was carried out in Goiânia, Brazil, using fruits from eight stock plants submitted to three pre-depulping treatments (control, fermentation and drying) and two depulping me- thods (industrial depulping and concrete-mixer with the addition of gravel). After the different pre-sowing processes, the fresh and dry pyrenes mass, remaining fibers adhered to the pyrene and seedling emergence were evaluated. The pulper removed an average of 45% more pyrene pulp than the concrete mixer. However, these methodologies did not result in differences in the emergence of plants, which was affected only by the pre-depulping treatment, with superiority in the use of fresh fruits. -

Análisis Aeropalinológico Del Parque Nacional El Palmar
Bol. Soc. Argent. Bot. 52 (3) 2017 N. E. Muñoz et al. - Análisis aeropalinológico del Parque NacionalISSN El0373-580 Palmar X Bol. Soc. Argent. Bot. 52 (3): 473-496. 2017 ANÁLISIS AEROPALINOLÓGICO EN TRES ÁREAS DE VEGETACIÓN DENTRO DEL PARQUE NACIONAL EL PALMAR (COLÓN, ENTRE RÍOS) Y SU RELACIÓN CON LA VEGETACIÓN LOCAL Y REGIONAL NADIA E. MUÑOZ1, MERCEDES DI PASQUO1, FERNANDO BIGANZOLI2 y WILLIAM B. BATISTA2,3 Summary: Aeropalinological analysis in three vegetation areas within El Palmar National Park (Colón, Entre Ríos) and its relationship with the local and regional vegetation. The diversity of pollen rain monthly collected during two years (2011-2013) from the atmosphere in Tauber traps located at three sites in El Palmar National Park (Entre Ríos Province) is used to characterize the source vegetation. Site 1 is a mixed area composed of grassland, palm savanna, and wetland communities, site 2 is a grassland area and site 3 is a dense palm savanna. A total of 71 pollen-grain types grouped in 43 families coming from local, regional and extra- regional areas are identified. Of them, sixteen pollen types with more than 1% of Annual Pollen Influx in at least two samples were used in this analysis. Different factors involved in quali-quantitave changes of taxa during the observation interval (e.g. pollination affinity, origin of pollen grains, canopy effect, meteorological variables) are further considered. The floral composition of each site compared to their palynoassemblages revealed that site 2 is characterized by a high abundance of Asteraeceae-Asteroideae, with an increase in the value of Vernonia (Asteraceae Cichoroidea) and Lamiaceae during the second year. -

What Is a Tree in the Mediterranean Basin Hotspot? a Critical Analysis
Médail et al. Forest Ecosystems (2019) 6:17 https://doi.org/10.1186/s40663-019-0170-6 RESEARCH Open Access What is a tree in the Mediterranean Basin hotspot? A critical analysis Frédéric Médail1* , Anne-Christine Monnet1, Daniel Pavon1, Toni Nikolic2, Panayotis Dimopoulos3, Gianluigi Bacchetta4, Juan Arroyo5, Zoltán Barina6, Marwan Cheikh Albassatneh7, Gianniantonio Domina8, Bruno Fady9, Vlado Matevski10, Stephen Mifsud11 and Agathe Leriche1 Abstract Background: Tree species represent 20% of the vascular plant species worldwide and they play a crucial role in the global functioning of the biosphere. The Mediterranean Basin is one of the 36 world biodiversity hotspots, and it is estimated that forests covered 82% of the landscape before the first human impacts, thousands of years ago. However, the spatial distribution of the Mediterranean biodiversity is still imperfectly known, and a focus on tree species constitutes a key issue for understanding forest functioning and develop conservation strategies. Methods: We provide the first comprehensive checklist of all native tree taxa (species and subspecies) present in the Mediterranean-European region (from Portugal to Cyprus). We identified some cases of woody species difficult to categorize as trees that we further called “cryptic trees”. We collected the occurrences of tree taxa by “administrative regions”, i.e. country or large island, and by biogeographical provinces. We studied the species-area relationship, and evaluated the conservation issues for threatened taxa following IUCN criteria. Results: We identified 245 tree taxa that included 210 species and 35 subspecies, belonging to 33 families and 64 genera. It included 46 endemic tree taxa (30 species and 16 subspecies), mainly distributed within a single biogeographical unit. -

Paysandisia Archon (Burmeister, 1879) - the Castniid Palm Borer (Lepidoptera, Castniidae) Chapter 14: Factsheets for 80 Representative Alien Species David Lees
Paysandisia archon (Burmeister, 1879) - The castniid palm borer (Lepidoptera, Castniidae) Chapter 14: Factsheets for 80 representative alien species David Lees To cite this version: David Lees. Paysandisia archon (Burmeister, 1879) - The castniid palm borer (Lepidoptera, Cast- niidae) Chapter 14: Factsheets for 80 representative alien species. Alien terrestrial arthropods of Europe, 4 (2), Pensoft Publishers, 2010, BioRisk, 978-954-642-555-3. hal-02928701 HAL Id: hal-02928701 https://hal.inrae.fr/hal-02928701 Submitted on 2 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 990 Edited by Alain Roques & David Lees / BioRisk 4(2): 855–1021 (2010) 14.65 – Paysandisia archon (Burmeister, 1879) - Th e castniid palm borer (Lepidoptera, Castniidae) Carlos Lopez-Vaamonde & David Lees Description and biological cycle: Large dayfl ying moth with clubbed antennae, wingspan 75–120 mm, upperside forewing greenish brown in both sexes, hindwing bright orange with a black band postdiscal to white spots (Photo left). Forewing underside orange, excepting beige tips. Upright fusiform eggs, about 4.7 mm. long and 1.5 mm wide, laid by the female’s extensible ovipositor between mid-June and mid-October. Fertile eggs pink, laid among palm crown fi bres, at the base of leaf rachis. -

Phytochemical Investigation of Phoenix Canariensis Hort. Ex Chabaud Leaves and Pollen Grains
Journal of Applied Pharmaceutical Science Vol. 6 (12), pp. 103-109, December, 2016 Available online at http://www.japsonline.com DOI: 10.7324/JAPS.2016.601214 ISSN 2231-3354 Phytochemical investigation of Phoenix canariensis Hort. ex Chabaud leaves and pollen grains Mohamed S. Hifnawy, Amr M. K. Mahrous, Rehab M. S. Ashour* Pharmacognosy Department, Faculty of pharmacy, Cairo University, Kasr El-Aini 11562, Egypt. ABSTRACT ARTICLE INFO Article history: Phoenix canariensis is a commonly grown, yet understudied, palm plant. The phytochemical screening of leaves Received on: 08/09/2016 and pollens revealed the presence of flavonoids, saponins, tannins, sterols and/or triterpenes. Quantitative Revised on: 09/10/2016 estimation of constituents, revealed that the total polyphenolics were higher in the leaves (69.9) than in pollens Accepted on: 22/11/2016 (29.98) expressed in mg gallic acid equivalent/g d.wt, the total flavonoids calculated as rutin equivalent were Available online: 28/12/2016 (23.86 mg/g) in leaves and (17.20 mg/g) in pollens, the total tannins content were 55.18 and 3.31 mg tannic acid equivalent/g fresh wt, while the total steroids content were 2.6 and 12.4 mg β-sitosterol equivalent/g d.wt, in Key words: leaves and pollens, respectively. Eighteen phenolic compounds and ten flavonoids were identified by HPLC. Phoenix canariensis, Lipids, GLC analysis of lipids, revealed the identification of phytosterols (4.93 and 28.90%), saturated (35.35 and Headspace volatiles, 40.56%) and unsaturated (62.42 and 59.01%) fatty acids in leaves and pollens, respectively. Proximate analysis Flavonoids, Phenolics, revealed a total moisture content of (6.4 and 7.7 %), crude fiber (32.22 and 39.50%), total ash (12.1 and 8.1%) Steroids. -

JIMENEZ-THESIS-2016.Pdf (685.2Kb)
IDENTIFYING AND CHARACTERIZING ROOSTS OF SOUTHERN AND NORTHERN YELLOW BATS (LASIURUS EGA AND LASIURUS INTERMEDIUS) A Thesis Presented to the Faculty of the College of Graduate Studies of Angelo State University In Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE by PATRICIA CITLALLY JIMENEZ May 2016 Major: Biology IDENTIFYING AND CHARACTERIZING ROOSTS OF SOUTHERN AND NORTHERN YELLOW BATS (LASIURUS EGA AND LASIURUS INTERMEDIUS) by PATRICIA CITLALLY JIMENEZ APPROVED: Dr. Loren K. Ammerman Dr. Robert C. Dowler Dr. Ben R. Skipper Dr. Biqing Huang April 5, 2016 APPROVED: Dr. Susan E. Keith Date Dean, College of Graduate DEDICATION This thesis is dedicated to my family, my future husband James Kiser, and my forever adorable yellow bats; “I can do all things through Him who gives me strength.” Using palm fronds as roosts, Yellow bats await. Hide-and-seek on the loose, Is the game that they play. iii ACKNOWLEDGMENTS I wish to start by thanking my thesis committee. I thank Dr. Ammerman for her never ending patience with my naivety and kookiness throughout this project, for her determination and knowledge to mold my skills to become a good researcher, and for teaching me how a strong work ethic, perseverance and a little creativity can lead to success. I thank Dr. Dowler for his reassurances and for always ensuring I produced quality work. I thank Dr. Skipper for being the best committee cheerleader a graduate student could ever hope for; without his guidance, understanding, and positive encouragement, I would still be stumbling through this project. And lastly, I’d like to thank Dr. -

Arizona Landscape Palms
Cooperative Extension ARIZONA LANDSCAPE PALMS ELIZABETH D AVISON Department of Plant Sciences JOHN BEGEMAN Pima County Cooperative Extension AZ1021 • 12/2000 Issued in furtherance of Cooperative Extension work acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, James A. Christenson, Director, Cooperative Extension, College of Agriculture and Life Sciences, The University of Arizona. The University of Arizona College of Agriculture and Life Sciences is an equal opportunity employer authorized to provide research, educational information and other services to individuals and institutions that function without regard to sex, race, religion, color, national origin, age, Vietnam Era Veteran's status, or disability. Contents Landscape Use ......................................... 3 Adaptation ................................................ 3 Planting Palms ......................................... 3 Care of Established Palms...................... 5 Diseases and Insect Pests ....................... 6 Palms for Arizona .................................... 6 Feather Palms ........................................... 8 Fan Palms................................................ 12 Palm-like Plants ..................................... 16 This information has been reviewed by university faculty. ag.arizona.edu/pubs/garden/az1121.pdf 2 The luxuriant tropical appearance and stately Adaptation silhouette of palms add much to the Arizona landscape. Palms generally can be grown below the 4000 ft level Few other plants are as striking in low and mid elevation in Arizona. However, microclimate may make the gardens. Although winter frosts and low humidity limit difference between success and failure in a given location. the choices somewhat, a good number of palms are Frost pockets, where nighttime cold air tends to collect, available, ranging from the dwarf Mediterranean Fan should be avoided, especially for the tender species. Palms palm to the massive Canary Island Date palm. -
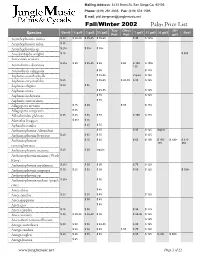
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Karyotype Analysis of Three Species of Sobal, L (Palmae: Coryphoideae)
_??_1992 The Japan Mendel Society Cytologia 57: 485-489, 1992 Karyotype Analysis of Three Species of Sobal, L (Palmae: Coryphoideae) Guadalupe Palomino and Hermilo J. Quero Jardin Botanico , Instituto de Biologia, Universidad Nacional, Autonoma de Mexico , Apdo. Post. 70-614, Mexico, 04510, D.F. Accepted May 26, 1992 Sabal is a New World genus which grows in the Northern Hemisphere from the Caribb ean Islands, Southern United States (USA), Central America, to Venezuela. Mexico has the greatest diversity of Sabal with 7 of the 15 known species (Zona 1990).A mong the genera of palms occurring in Mexico, Sabal is one of the most economically i mportant genus, all of its species are intensively utilized by the rural population. The mature l eaves are used for thatching , the young leaves are used for make different kind of handicrafts, trunks for constructions . Sabal mexicana is also used as a source of edible "palm heart" and their fruits are used as complementary pig fodder (Caballero 1991). Four of the 7 Sabal species that occur in Mexico grow in the Yucatan Peninsula. They are, Sabal mexicana, S. mauritiiformis, S. yapa and the recently described species S. gretheriae. The three former species are clearly distinctive but S. gretheriae is closely similar to S. mexicana (Quero 1991). Palm chromosome counts have been reported for 111 genera and approximately 250 spec ies. The presence of a consecutive gametic numbers ranging from n=13 to n=18 , is called a d ysploid series. Palms of the subfamily Coryphoideae are considered to be the most primitive group with n=18 (Uhl and Druansfield 1987). -

Palm Trees for Landscapes in Tulare & Kings Counties
Palm Trees for Landscapes in Tulare & Kings Counties Suggested by Nancy Gravender, UC Master Gardener MEDIUM-SIZED PALM TREES (10-25 Ft. Tall) FAN PALMS: Guadalupe Palm (Brahea edulis) – Grows to 20 ft., spread 15 ft., solitary trunk, large handsome fan leaves, (old leaves self-prune), slow growth, temperature range 20-105 °F. Mexican Blue Palm (Brahea armata) – Grows to 20 ft., spread 10 ft., solitary trunk, stiff, palmate fan covered with pale blue bloom, slow growth, temp. range 20-120 °F. Mediterranean or European Fan Palm (Chamerops humilus) – Grows to 15 ft., spread 15 ft., clumping or multiple trunks, 4-5 ft. in diameter; fan, 2-3 ft. diameter stiff leaflets, petiole has sharp spines, slow growth, temp. range 20-120 °F. Windmill Palm (Trachycarpus fortunei) – Grows to 15 ft. spread 5 ft., solitary trunk covered with old leaf bases, and brown fibrous matted hairy fibers; fan irregularly divided, if not trimmed, the old leaves hang down; temp. range 10–115 °F. FEATHER PALMS: Pigmy Date Palm (Phoenix roebelinii) – Grows to 10 ft., solitary trunk, 4-8 in. in diameter, feather with leaflets much softer than those of other Phoenix palms, although the lower leaflets still contain sharp spines, slow growth, temperature range 28-105 °F. Requires shade in this area. Pindo Palm (Butia capitata) - Grows to 20 ft., spread 15 ft., solitary trunk, blue green pinnate feather, slow growth, temp. range 15-120 °F. Queen Palm (Syagrus romanzoffiana) – Grows to 25 ft. spread 12 ft., solitary trunk ringed with old leaf bases, feather, plumose (leaflets radiating at different angles), fast growth with abundant summer water and fertilizer, temp. -

Trachycarpus Fortunei) and Its Application Potential
Article Anatomy of the Windmill Palm (Trachycarpus fortunei) and Its Application Potential Jiawei Zhu 1, Jing Li 1,2, Chuangui Wang 3 and Hankun Wang 1,* 1 Department of Biomaterials, International Center for Bamboo and Rattan, State Forestry Administration and Beijing Co-Built Key Lab for Bamboo and Rattan Science & Technology, Beijing 100102, China; [email protected] (J.Z.); [email protected] (J.L.) 2 Key Lab of Wood Science and Technology of National Forestry and Grassland Administration, Research Institute of Wood Industry, Chinese Academy of Forestry, Beijing 100102, China 3 School of Forestry & Landscape Architecture, Anhui Agricultural University, Hefei 230036, China; [email protected] * Correspondence: [email protected] Received: 13 November 2019; Accepted: 9 December 2019; Published: 10 December 2019 Abstract: The windmill palm (Trachycarpus fortunei (Hook.) H. Wendl.) is widely distributed and is an important potential source of lignocellulosic materials. The lack of knowledge on the anatomy of the windmill palm has led to its inefficient use. In this paper, the diversity in vascular bundle types, shape, surface, and tissue proportions in the leaf sheaths and stems were studied with digital microscopy and scanning electron microscope (SEM). Simultaneously, fiber dimensions, fiber surfaces, cell wall ultrastructure, and micromechanics were studied with atomic force microscopy (AFM) and a nanoindenter. There is diversity among vascular bundles in stems and leaf sheaths. All vascular bundles in the stems are type B (circular vascular tissue (VT) at the edge of the fibrous sheath (FS)) while the leaf sheath vascular bundles mostly belong to type C (aliform (VT) at the center of the (FS), with the wings of the (VT) extending to the edge of the vascular bundles). -

Coconut and Other Palm Trees Posted on August 8, 2019 by Leslie Lang
HOME HOURS & DIRECTIONS GARDEN SLIDESHOW GARDEN NEWS & BLOG Coconut and Other Palm Trees Posted on August 8, 2019 by Leslie Lang Of all the types of palm trees, many people here in Hawai‘i are most familiar with the coconut palm, Cocos nucifera. It’s the tree that says, “tropics.” But there’s so much more to the coconut palm. Its fruit, the niu or coconut, is so useful that early Polynesians brought it along to sustain themselves when they sailed across the Pacific to Hawai‘i. Polynesians knew that when they settled on new islands, they could plant coconuts and make use of the entire tree that grew—not only the coconut meat and water, but also the leaves, the wood, the fiber, and every other part. According to the book Canoe Plants of Ancient Hawaii, “Besides drink, food and shade, niu offers the possibilities of housing, thatching, hats, baskets, furniture, mats, cordage, clothing, charcoal, brooms, fans, ornaments, musical instruments, shampoo, containers, implements and oil for fuel, light, ointments, soap and more.” The only palm tree that’s native to Hawai‘i is the loulu (Pritchardia). There are perhaps 19 loulu species in Hawai‘i and a few related species in Tahiti and Fiji. Hawai‘i used to have large loulu forests, but while some loulu still survive in the wild, many disappeared because of rats, pigs, goats, and even people. Within the genus Pritchardia, there are 25 species of palms native to the tropical Pacific Islands. In Hawai‘i, as many as 19 species of Pritchardia are endemic, and some of them are categorized as endangered, rare, or vulnerable.