Dosage Forms, Routes of Administration, and Dispensing Medications 81
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inhalation Devices: Various Forms of Administration for Therapeutic Optimization
vv ISSN: 2640-8082 DOI: https://dx.doi.org/10.17352/oja CLINICAL GROUP Renata Cristina de Angelo Calsaverini Leal* Review Article Santa Fé do Sul Foundation of Education and Culture, Brazil Inhalation Devices: Various forms Dates: Received: 31 May, 2017; Accepted: 26 June, of administration for Therapeutic 2017; Published: 27 June, 2017 *Corresponding author: Renata Cristina de Angelo Optimization Calsaverini Leal, Santa Fé do Sul Foundation of Education and Culture, Brazil, Tel: 55 (17) 3272-2769, E-mail: Keywords: Inhalation; Aerosol; Nebulizer Summary https://www.peertechz.com Introduction: Aerosol therapy consists of spraying liquid particles suspended for therapeutic purposes in the respiratory tract. With direct absorption and deposition at the lung level, avoiding side effects and presenting fast response time. Several factors infl uence the drug action, such as size, particle movement, ventilatory fl ow, pulmonary expansion, anatomy, respiratory mechanics and the nebulizer and patient interface. The therapeutic optimization depends on the type of nebulizer differentiating itself by the physical principle that generates the mist. Objectives: Check advantages and disadvantages of different inhalation devices. Methodology. This is a review of the PubMed database using descriptors: ultrasonic and jet nebulizer, aerosol deposition in the lung, metered dose inhaler and dry, inhaler therapy. Results: Different devices are mentioned in the literature: pneumatic and ultrasonic nebulizers (administering solutions), metered pressurized inhalers - pMDI used with or without expander chamber (administering suspensions) and dry powder inhalers - DPI (administering powder). Discussion and Conclusion: The US has advantages: quiet, does not require coordinating abilities, without propellant gases and quick nebulization with small amount of solution. Disadvantages: change in the active principle of thermosensitive drugs, deposition in the oropharynx and VAI of 2% of inhaled particles. -
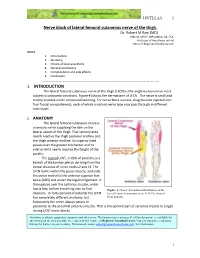
Nerve Block of Lateral Femoral Cutaneous Nerve of the Thigh
18VTLLAA 1 Nerve block of lateral femoral cutaneous nerve of the thigh. Dr. Robert M Raw (MD) . MBChB, MFGP, MPraxMed, DA, FCA. Professor of Anesthesia retired Editor of Regional-Anesthesia.Com INDEX. 1. Introduction 2. Anatomy 3. Choice of local anesthetic 4. General indications 5. Complications and side effects 6. Conclusion ------------------------------------------------------------------------------------ 1. INTRODUCTION The lateral femoral cutaneous nerve of the thigh (LFCN) is the single human nerve most subject to anatomic variations. Figure #1shows the dermatome of LFCN. The nerve is small and mostly invisible under ultrasound scanning. For nerve block success, drug must be injected into four fascial compartments, each of which a variant nerve type may pass through in different individuals. 2. ANATOMY The lateral femoral cutaneous nerve is a sensory nerve supplying the skin on the lateral aspect of the thigh. That sensory area nearly reaches the thigh posterior midline and the thigh anterior midline. Its superior limit passes over the greater trochanter and its inferior limit nearly reaches the height of the patella. The typical LCNT, in 60% of patients, is a branch of the lumbar plexus deriving from the dorsal divisions of nerve roots L2 and L3. The LFCN forms within the psoas muscle, and exits the pelvis medial to the anterior superior iliac spine (ASIS) and under the inguinal ligament. It then passes over the sartorius muscle, under fascia lata, before branching into its final Figure 1. Classic dermatomal distribution of the divisions. In forty percent of patients the LFCN lateral femoral cutaneous nerve (LFCN), derived has completely different anatomy, but from Sobotta. fortunately the nerve always passes in proximity to the proximal sartorius muscle. -

Inhaled Steroids in Asthma a Patient's Guide
I I v v v v d v v Patient advice and liaison service (PALS) Actions r v If you have a compliment, complaint or It is sensible to ensure you make the r concern please contact our PALS team on following steps now you have a new r 020 7288 5551 or inhaler d [email protected] d d Pick up our guide on inhalers and If you need a large print, audio or spacers to complement this leaflet translated copy of this leaflet please Inhaled Steroids in Agree on a personalised asthma contact us on 020 7288 3182. We will try plan (this is done with your doctor or our best to meet your needs. Asthma nurse and usually written down for future reference) Remember to take your medicines A patient’s guide as advised Should your inhalers fail to relieve your symptoms, go straight to Accident and Emergency Need Help? Whittington Paediatric Asthma Nurse Tel: 020 7288 5527 Whittington Health Community Nurse Magdala Avenue Islington 020 3316 1950 (8am-6pm) London N19 5NF Haringey 020 8887 3301 (9am – 5pm) Phone: 020 7272 3070 www.whittington.nhs.uk Asthma UK 0300 222 5800 Date published: 25/09/2018 www.asthma.org.uk Review date: 25/09/2020 Ref: C&YP/Paed/ISA/03 © Whittington Health Please recycle Tel: 020 7272 3070 Asthma There are many types of preventer Side Effects Asthma is a common condition affecting inhaler. There are simple steroids like Parents worry about children and young the airway. Usually a trigger (such as dust beclomethasone, and then there are also adults taking inhaled steroids because of or pollen) irritates the airways which combined inhalers, called seretide or side effects they’ve heard about. -

Gel-Syn™ Product Information Caution
GEL-SYN™ PRODUCT INFORMATION CAUTION: Federal law restricts this device to sale by or on the order of a physician (or properly licensed practitioner). CONTENT Each 1 mL of Gel-Syn contains: Sodium Hyaluronate: 8.4 mg Sodium Chloride: 8.5 mg Sodium Phosphate, Dibasic: 0.16 mg Sodium Phosphate, Monobasic: 0.045 mg Water for Injection: q.s. to 1.0 mL DESCRIPTION Gel-Syn is a sterile, buffered solution of highly purified sodium hyaluronate with a molecular weight of approximately 1100 kDa, obtained through fermentation of Streptococci of Lancefield groups A and C and chemically unmodified. INDICATION Gel-Syn is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy and simple analgesics (e.g., acetaminophen). CONTRAINDICATIONS • Do not administer to patients with known hypersensitivity (allergy) to sodium hyaluronate preparations. • Do not inject Gel-Syn into the knees of patients having knee joint infections or skin diseases or infections in the area of the injection site. WARNINGS • Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because sodium hyaluronate can precipitate in their presence. • Inject into the synovial space only. Do not inject by intravascular route. • Do not inject outside the synovial space or into the synovial tissue or capsule. An extra- articular injection of the product can cause local adverse events. PRECAUTIONS General • The safety and effectiveness of Gel-Syn in locations other than the knee, and for conditions other than osteoarthritis, have not been established. • Strict aseptic administration technique must be followed. -

Pricing Sheet
Trusted Service. Proven Savings. Pharmacy: (325) 704-5222 Fax: (325) 777-4819 17 Windmill Circle Suite B Abilene, TX 79606 bigcountry.clarxpharmacy.com SureScript ID: 1171544616 Dermatology Compounds UPDATED 9/3/2020 Medication QTY Price Fluorouracil/ Calcipotriene Cream 4.5/ 0.005% 30 g $55 Fluorouracil Cream 4.5% or 1.5% 30 g $50 Wart Peel Cream (Salicylic Acid/ Fluorouracil 17/ 2%) 30 g $45 Ivermectin Lotion 1.5% 30 g $45 Double Rosacea Cream (Metronidazole/ Ivermectin 1/ 1%) 30 g $55 Triple Rosacea Cream (Azelaic Acid/ Metronidazole/ Ivermectin 15/ 1/ 1%) 30 g $45 Azelaic Acid Cream 17% 30 g $45 Calcipotriene/ Clobetasol Cream 0.005/ 0.045% 60 g $85 Clioquinol/ Hydrocortisone Cream 1/ 1% 30 g $55 Tretinoin Micro Cream 0.033/ 0.055/ 0.09% 45 g $55 Clindamycin/ Tretinoin Micro Cream 1/0.03% 30 g $75 Triamcinolone/ Hydroquinone/ Tretinoin Cream 0.025/ 4/ 0.05% 30 g $55 Hydrocortisone/ Hydroquinone/ Tretinoin Cream 2.5/ 8/ 0.05% 30 g $55 Hydroquinone/ Kojic Acid Cream 12/ 6% or 10/ 3% 30 g $65 Hydroquinone/ Tretinoin Cream 4/ 0.05% 30 g $65 Squaric Acid Solution 0.1 / 0.01 / 0.001% 15 ml $85 Urea Cream 35% 90 g $45 Dapsone 6% Cream 60 g $55 Costmetic Numbing Oint. (Lidocain 23%/Tetracain 7%) 60g $35 General topical compound pricing for other compounds QTY Price Variations to the compounds above will fall into the pricing below. One Active Ingredient 30g $79 Two Active Ingredients 30g $89 Three Active Ingredients 30g $99 *This applies to most compounds (90%) not all, if exceptionally expensive we will call the office to discuss* All cash, no rebates or insurance to mess with! Medicare patients get medications at these great prices too! Free Delivery in 3-5 business days for compounds Page 1 Trusted Service. -

Absorbine Veterinary Liniment for Horses
Doc# 03.287 Ver. 11 SAFETY DATA SHEET ABSORBINE® VETERINARY LINIMENT SECTION 1 - PRODUCT AND COMPANY IDENTIFICATION 1.1 Trade Name (as labeled): Absorbine® Veterinary Liniment Synonyms: N/A CAS No: Mixture 1.2 Product Use: Soothes sore muscles and stiff joints 1.3 Company Name: W.F. Young Company Address: 302 Benton Dr Company Address Cont: East Longmeadow, MA 01028 Business Phone: ( 413) 526-9999 Website: www.wfyoung.com 1.4 Emergency Telephone Number: (413) 526-9999 Date of Current Revision: January 17, 2017 Date of Last Revision: August 7, 2015 SECTION 2 - HAZARD IDENTIFICATION EMERGENCY OVERVIEW: This product is a green thin liquid with an acetone odor. Health Hazards: May cause skin, eye, and respiratory system irritation. Flammabilit Hazards: This product is a flammable liquid with a flash point over 20°F (-6. 7°C). Reactivit Hazards: None. Environmental Hazards: The environmental effectsof this product have not been investigated, however release may cause long term adverse environmental effects. US DOT Symbols: EU and GHS Symbols: Signal Word: Danger! 2.1 CLASSIFICATION OF SUBSTANCE OR MIXTURE IN ACCORDANCE WITH 29 CFR 1200 (OSHA HCSl AND THE EUROPEAN UNION DIRECTIVES: This product does meet the definition of a hazardous substance or preparation as defined by 29 CFR 1910. 1200 or the European Union Council Directives 67 /548/EEC, 1999/45/EC, 1272/2008/EC and subsequent Directives. EU HAZARD CLASSIFICATIONOF INGREDIENTS PER DIRECTIVE 1272/2008/EC: IndexNumber: EC# 201-939-0 This substance is not classified in the AnnexVI of Directive 67/548/EEC EC# 200-662-2 This substance is classified in the AnnexVI of Directive 67/548/EEC Index# 606-001-00-8 Substances not listed either individually or in group entries must be self classified. -

Physical-Chemical Characteristics of Whitening Toothpaste and Evaluation of Its Effects on Enamel Roughness
Dental materials Physical-chemical characteristics of whitening toothpaste and evaluation of its effects on enamel roughness Sérgio Paulo Hilgenberg(a) Abstract: This in vitro study evaluated the physical-chemical characteris- (a) Shelon Cristina Souza Pinto tics of whitening toothpastes and their effect on bovine enamel after ap- Paulo Vitor Farago(b) Fábio André Santos(a) plication of a bleaching agent (16% carbamide peroxide). Physical-chem- Denise Stadler Wambier(a) ical analysis was made considering mass loss by desiccation, ash content and pH of the toothpastes. Thirty bovine dental enamel fragments were prepared for roughness measurements. The samples were subjected to (a) Department of Dentistry, School of Dentistry, Ponta Grossa State University, bleaching treatments and simulated brushing: G1. Sorriso Dentes Brancos Ponta Grossa, PR, Brazil. (Conventional toothpaste), G2. Close-UP Whitening (Whitening tooth- (b) Department of Pharmacy, School of paste), and G3. Sensodyne Branqueador (Whitening toothpaste). The av- Dentistry, Ponta Grossa State University, erage roughness (Ra) was evaluated prior to the bleaching treatment and Ponta Grossa, PR, Brazil. after brushing. The results revealed differences in the physical-chemical characteristics of the toothpastes (p < 0.0001). The final Ra had higher values (p < 0.05) following the procedures. The mean of the Ra did not show significant differences, considering toothpaste groups and bleach- ing treatment. Interaction (toothpaste and bleaching treatment) showed significant difference -

An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: a Review
Muthadi Radhika Reddy /J. Pharm. Sci. & Res. Vol. 12(7), 2020, 925-940 An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: A Review Muthadi Radhika Reddy1* 1School of pharmacy, Gurunanak Institute of Technical Campus, Hyderabad, Telangana, India and Department of Pharmacy, Gandhi Institute of Technology and Management University, Vizag, Andhra Pradesh, India INTRODUCTION 2. Useful in situations where rapid onset of action Fast dissolving drug delivery systems were first developed required such as in motion sickness, allergic attack, in the late 1970s as an alternative to conventional dosage coughing or asthma forms. These systems consist of solid dosage forms that 3. Has wide range of applications in pharmaceuticals, Rx disintegrate and dissolve quickly in the oral cavity without Prescriptions and OTC medications for treating pain, the need of water [1]. Fast dissolving drug delivery cough/cold, gastro-esophageal reflux disease,erectile systems include orally disintegrating tablets (ODTs) and dysfunction, sleep disorders, dietary supplements, etc oral thin films (OTFs). The Centre for Drug Evaluation [4] and Research (CDER) defines ODTs as,“a solid dosage 4. No water is required for the administration and hence form containing medicinal substances which disintegrates suitable during travelling rapidly, usually within a matter of seconds, when placed 5. Some drugs are absorbed from the mouth, pharynx upon the tongue” [2]. USFDA defines OTFs as, “a thin, and esophagus as the saliva passes down into the flexible, non-friable polymeric film strip containing one or stomach, enhancing bioavailability of drugs more dispersed active pharmaceutical ingredients which is 6. May offer improved bioavailability for poorly water intended to be placed on the tongue for rapid soluble drugs by offering large surface area as it disintegration or dissolution in the saliva prior to disintegrates and dissolves rapidly swallowing for delivery into the gastrointestinal tract” [3]. -

Preparation and Characterization of Oil-In-Water and Water-In-Oil Emulsions
1 Preparation and Characterization of Oil-in-Water and Water-in-Oil Emulsions Prepared For Dr. Reza Foudazi, Ph.D. Chemical and Materials Engineering New Mexico State University By Muchu Zhou May 10, 2016 2 1 Introduction 1.1 Purpose of This Report The objective of this report is to clarify what I have done this semester for research course CHME 498. The research interest is “Preparation and Characterization of Oil-in-Water and Water-in-Oil Emulsions”. Thus, I would like to talk about what is emulsion, what are the main characteristics of emulsions, what are the existing methods for preparations of emulsions and how to make simple emulsions. 1.2 Background of This Report Emulsion is a kind of mixture comprised of two or more liquids, which usually are immiscible, and surfactant. The common types of emulsions are oil-in-water emulsion and water-in-oil emulsion. According to Aronson (1988), the emulsions have important industrial value in the wide range of field and it has been studied extensively recently. The emulsions play an important role in the industrial production and it has been applied to many fields including food industry, cosmetics industry and pharmaceutical industry. In the food industry, emulsifier can function as dough conditioners in order to improve tolerance to variations in flour and other ingredient quality. In the cosmetic industry, the majority of facial creams and lotions are emulsions. 1.3 Scope of This Report 3 This report is going to cover the following contents. Introduction of emulsions. Effect of surfactant. Common materials for preparation of emulsions. -

The Toxicology of Glycol Ethers and Its Relevance to Man (Fourth Edition) Volume I
The Toxicology of Glycol Ethers and its Relevance to Man (Fourth Edition) Volume I Technical Report No. 95 ISSN-0773-8072-95 Brussels, February 2005 The Toxicology of Glycol Ethers and its Relevance to Man ECETOC TECHNICAL REPORT No. 95 © Copyright – ECETOC AISBL European Centre for Ecotoxicology and Toxicology of Chemicals 4 Avenue E. Van Nieuwenhuyse (Bte 6), B-1160 Brussels, Belgium. All rights reserved. No part of this publication may be reproduced, copied, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the copyright holder. Applications to reproduce, store, copy or translate should be made to the Secretary General. ECETOC welcomes such applications. Reference to the document, its title and summary may be copied or abstracted in data retrieval systems without subsequent reference. The content of this document has been prepared and reviewed by experts on behalf of ECETOC with all possible care and from the available scientific information. It is provided for information only. ECETOC cannot accept any responsibility or liability and does not provide a warranty for any use or interpretation of the material contained in the publication. ECETOC TR No. 95 The Toxicology of Glycol Ethers and its Relevance to Man The Toxicology of Glycol Ethers and its Relevance to Man CONTENTS - VOLUMES I AND II EXECUTIVE SUMMARY 1 SUMMARY AND CONCLUSIONS 3 Recommendations for further work 13 1. INTRODUCTION 14 1.1 Conversion factors and physico-chemical properties 14 1.2 Production and use 14 1.2.1 Manufacture of ethylene-series glycol ethers 14 1.2.2 Manufacture of propylene-series glycol ethers 15 1.2.3 Uses 15 2. -

Inhalation Solution, USP for Oral Inhalation Use What Is Tobramycin
PATIENT INFORMATION TOBRAMYCIN (TOE-BRA-MYE-SIN) Inhalation Solution, USP for oral inhalation use What is Tobramycin Inhalation Solution? Tobramycin inhalation solution is a prescription medicine that is used to treat people with cystic fibrosis who have a bacterial infection called Pseudomonas aeruginosa. Tobramycin inhalation solution contains an antibacterial medicine called tobramycin (an aminoglycoside). It is not known if tobramycin inhalation solution is safe and effective: in children under 6 years of age in people who have an FEV1 less than 25% or greater than 75% predicted in people who are colonized with a bacterium called Burkholderia cepacia Do not take tobramycin inhalation solution if you are allergic to tobramycin, any of the ingredients in tobramycin inhalation solution, or to any other aminoglycoside antibacterial. See the end of this Patient Information for a complete list of ingredients in tobramycin inhalation solution. Before you take tobramycin inhalation solution, tell your healthcare provider about all of your medical conditions, including if you: have or have had hearing problems (including noises in your ears such as ringing or hissing) have dizziness have or have had kidney problems have or have had problems with muscle weakness such as myasthenia gravis or Parkinson’s disease have or have had breathing problems such as wheezing, coughing, or chest tightness are pregnant or plan to become pregnant. Tobramycin inhalation solution is in a class of drugs that can harm your unborn baby. are breastfeeding or plan to breastfeed. It is not known if tobramycin passes into your breast milk. are receiving aminoglycoside therapy by injection or through a vein (intravenous) while taking tobramycin inhalation solution. -

Cream LOTRISONE® Lotion (Clotrimazole and Betamethasone Dipropionate)
LOTRISONE® Cream LOTRISONE® Lotion (clotrimazole and betamethasone dipropionate) FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE. NOT RECOMMENDED FOR PATIENTS UNDER THE AGE OF 17 YEARS AND NOT RECOMMENDED FOR DIAPER DERMATITIS. DESCRIPTION LOTRISONE® Cream and Lotion contain combinations of clotrimazole, a synthetic antifungal agent, and betamethasone dipropionate, a synthetic corticosteroid, for dermatologic use. Chemically, clotrimazole is 1–(o-chloro-α,α-diphenylbenzyl) imidazole, with the empirical formula C22H17CIN2, a molecular weight of 344.84, and the following structural formula: Clotrimazole is an odorless, white crystalline powder, insoluble in water and soluble in ethanol. Betamethasone dipropionate has the chemical name 9-fluoro-11β,17,21- trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate, with the empirical formula C28H37FO7, a molecular weight of 504.59, and the following structural formula: 1 LRN# 000370-LOS-MTL-USPI-1 Betamethasone dipropionate is a white to creamy white, odorless crystalline powder, insoluble in water. Each gram of LOTRISONE Cream contains 10 mg clotrimazole and 0.643 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone), in a hydrophilic cream consisting of purified water, mineral oil, white petrolatum, cetyl alcohol plus stearyl alcohol, ceteareth-30, propylene glycol, sodium phosphate monobasic monohydrate, and phosphoric acid; benzyl alcohol as preservative. LOTRISONE Cream may contain sodium hydroxide. LOTRISONE Cream is smooth, uniform, and white to off-white in color. Each gram of LOTRISONE Lotion contains 10 mg clotrimazole and 0.643 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone), in a hydrophilic base of purified water, mineral oil, white petrolatum, cetyl alcohol plus stearyl alcohol, ceteareth-30, propylene glycol, sodium phosphate monobasic monohydrate, and phosphoric acid; benzyl alcohol as a preservative.