Obstetrical Bleeding in Second Half of Pregnancy, in Labor and During Postpartum Term
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heterotopic Cervical Pregnancy
Elmer ress Case Report J Clin Gynecol Obstet. 2015;4(4):307-311 Heterotopic Cervical Pregnancy Mathangi Thangavelua, b, Ravinder Kalkata Abstract tenderness or cervical excitation. Initial hormonal investiga- tions showed BHCG levels were raised to 17,276 IU and ini- We report a rare case of heterotopic cervical pregnancy, which posed tial ultrasound was suggestive of minimal retained products diagnostic challenge. With increasing IVF treatment and raising ce- of conception (Fig. 1). However, a repeat BHCG showed an sarean section rate, there is increasing incidence for non-tubal hetero- increasing trend reaching up to 29,971 IU in 96 h. A repeat topic pregnancy. We have discussed the clinical course of our case, transvaginal scan showed the endometrial cavity had mixed diagnosis and management of cervical pregnancy and some good echoes and multiple cystic spaces, largest measuring 6 × 7 × medical practices to avoid missing atypical presentations of ectopic 8 mm with color flow suggesting a possible molar pregnancy pregnancy. (Fig. 2). Bilateral ovarian cysts were present in both adnexa. Laparoscopy and dilatation and curettage were arranged Keywords: Cervical pregnancy; Heterotopic; Ectopic in view of high BHCG levels and no clear evidence of intrau- terine pregnancy. Laparoscopy was negative for tubal ectopic pregnancy and dilatation and curettage was performed. Post- operatively BHCG levels were monitored to ensure its levels were declining. The levels initially dropped to 2,611 IU from Introduction 29,971 IU in a week after D&C. However, the subsequent BHCG levels doubled to 4,207 IU 2 weeks after D&C. With We report an extremely rare case of spontaneous heterotopic the knowledge of earlier scan findings, raising BHCG levels cervical pregnancy who needed multiple investigations before raised the concern of persistent trophoblastic disease. -

Case 1: Postpartum Hemorrhage Secondary to Uterine Atony
Case 1: Postpartum Hemorrhage Secondary to Uterine Atony Learning Objectives By the end of this scenario, each care team member should be able to successfully do the following: ▪ Recognize risk factors for postpartum hemorrhage. ▪ Identify postpartum hemorrhage due to uterine atony and be able to treat with appropriate medical management. ▪ Demonstrate teamwork and communication skills during a simulated postpartum hemorrhage. Planned Completion Points To successfully complete this scenario, the care team should successfully do the following: ▪ Recognize uterine atony as the etiology for postpartum hemorrhage. ▪ Perform uterine massage. ▪ Administer two different uterotonic medications. ▪ Call for blood (e.g. 2 units of PRBCs). OR Page | 1 © 2019 American College of Obstetricians and Gynecologists ▪ If 10 minutes has elapsed after recognition of hemorrhage and the team has not corrected the hemorrhage or called for blood. Expected Duration Approximately 60 minutes (30 minutes for simulation / 30 minutes for debriefing). Case Scenario Patient: Marla Smith Mrs. Marla Smith is a 38-year-old G3P2012 who was admitted in active labor at 39+3 weeks and had a spontaneous vaginal delivery 30 minutes ago. Her delivery was uncomplicated. She had a first-degree laceration that did not require repair. She is approximately 30 minutes postpartum and has just called out because she feels dizzy and has more bleeding. Patient Information ▪ She has no significant past medical history. ▪ She has no known drug allergies. ▪ Her pregnancy was uncomplicated except for an elevated 1-hour glucose screen with a normal 3- hour glucose tolerance test. Laboratory Data (On Admission): ▪ Hemoglobin: 12.2 ▪ Hematocrit: 36.6 ▪ WBC: 12,000 ▪ Platelets: 218,000 Delivery Information ▪ Measurement of cumulative blood loss (as quantitative as possible) from the delivery was 300cc. -
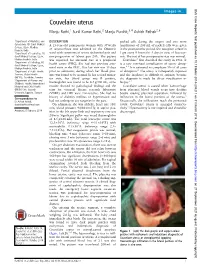
Couvelaire Uterus Manju Rathi,1 Sunil Kumar Rathi,2 Manju Purohit,3,4 Ashish Pathak2,5
BMJ Case Reports: first published as 10.1136/bcr-2014-204211 on 31 March 2014. Downloaded from Images in… Couvelaire uterus Manju Rathi,1 Sunil Kumar Rathi,2 Manju Purohit,3,4 Ashish Pathak2,5 1Department of Obstetrics and DESCRIPTION packed cells during the surgery and two more Gynecology, RD Gardi Medical A 23-year-old primiparous woman with 37 weeks transfusions of 200 mL of packed cells were given College, Ujjain, Madhya Pradesh, India of amenorrhoea was admitted to the Obstetric in the postoperative period. She was given cefazolin 2Department of Peadiatrics, RD ward with symptoms of severe abdominal pain and 1 gm every 8 hours for 5 days in view of leucocyt- Gardi Medical College, Ujjain, non-progression of labour past 20 h. The patient osis. The rest of her postoperative stay was normal. Madhya Pradesh, India 1 fi 3 was registered for antenatal care at a peripheral Couvelaire rst described the entity in 1911. It Department of Pathology, RD health centre (PHC). She had two previous ante- is a rare non-fatal complication of severe abrup- Gardi Medical College, Ujjain, 23 Madhya Pradesh, India natal visits at the PHC. Her last visit was 15 days tion. It is estimated to complicate 5% of all cases 2 4Department of Public Health prior to admission, during which her blood pres- of abruption. The entity is infrequently reported Sciences, Global Health sure was found to be normal. In her second trimes- and the incidence is difficult to estimate because (IHCAR), Stockholm, Sweden 5 ter visit, her blood group was B positive, the diagnosis is made by direct visualisation or Department of Women and 23 Children’s Health, International haemoglobin was found to be 8.5 g/100 mL, urine biopsy. -

Successful Treatment of Cervical Ectopic Pregnancy with Multi Dose
Case Report iMedPub Journals Gynaecology & Obstetrics Case report 2020 www.imedpub.com ISSN 2471-8165 Vol.6 No.2:14 DOI: 10.36648/2471-8165.6.2.94 Successful Treatment of Cervical Ectopic Iqbal S1*, Iqbal J2, Nowshad N1 and Pregnancy with Multi Dose Methotrexate Mohammad K1 Therapy 1 Department of Obstetrics and Gynecology, Latifa Hospital, Dubai Health Authority Jaddaf, Dubai, UAE 2 Department of Medical Education, Dubai Abstract Medical University, Dubai, UAE Cervical ectopic pregnancies account for less than 1% of all pregnancies. Earlier, it was associated with significant hemorrhage and was treated presumptively with hysterectomy. With the advent of enhanced ultrasound techniques, early *Corresponding author: Iqbal S detection of these pregnancies has led to the development of more effective conservative management. We present a case of a cervical ectopic pregnancy successfully treated with multi-dose Methotrexate therapy. [email protected] A 37-year-old lady, G3P0+2, pregnant for 9 weeks and 4 days, presented with bleeding per vagina, mild lower abdomen and back pain. Serum Beta-hCG done Department of Obstetrics and Gynecology, 5 days ago was 950 mIU/mL. She was diagnosed as ectopic cervical pregnancy Latifa Hospital, Dubai Health Authority by clinical examination which was confirmed by transvaginal ultrasonography Jaddaf, Dubai, UAE. and subsequently managed by Methotrexate (MTX) Hybrid double dose protocol. Due to rising Beta-hCG and continuous bleeding, it was modified to Multi dose Tel: 971569400124 Methotrexate Therapy. Thereafter, the patient was asymptomatic with falling beta-hCG and she was put on a weekly follow up in the clinic. Keywords: Ectopic pregnancy; Cervical pregnancy; Methrotrexate; Gynaecology Citation: Iqbal S, Iqbal J, Nowshad N, Mohammad K (2020) Successful Treatment of Cervical Ectopic Pregnancy with Multi Received: March 31, 2020; Accepted: May 02, 2020; Published: May 06, 2020 Dose Methotrexate Therapy. -

Alcohol, Breastfeeding, and Development at 18 Months
Alcohol, Breastfeeding, and Development at 18 Months Ruth E. Little, ScD*; Kate Northstone, MSc‡; Jean Golding, PhD, ScD‡; and the ALSPAC Study Team‡ ABSTRACT. Objective. We aimed to replicate a pre- literature search, we found only 1 systematic study1 vious study of 1-year-olds that reported a deficit in motor that measured drinking during lactation and subse- development associated with moderate alcohol use dur- quent child development. The authors reported a ing lactation, using a different but comparable popula- decrease in the motor development of infants at 1 tion. year of age, as measured by the Bayley Scales,2 when Methodology. The mental development of 915 18- the lactating mother had as little as 1 drink daily in month-old toddlers from a random sample of a longitu- dinal population-based study in the United Kingdom the first 3 months after delivery. This result was was measured using the Griffiths Developmental Scales. independent of drinking during pregnancy. Frequent self-administered questionnaires during and Lactation is the preferred method of early infant after pregnancy provided maternal data. The dose of feeding, and drinking alcohol is a common social alcohol available to the lactating infant was obtained by custom in most of the western world. Knowing the multiplying the alcohol intake of the mother by the pro- effect on the infant when lactation and alcohol drink- portion of breast milk in the infant’s diet. We compared ing are combined is of high importance, especially in this dose with the Griffiths Scales of Mental Develop- view of the previous report of its adverse effects. -

A Guide to Obstetrical Coding Production of This Document Is Made Possible by Financial Contributions from Health Canada and Provincial and Territorial Governments
ICD-10-CA | CCI A Guide to Obstetrical Coding Production of this document is made possible by financial contributions from Health Canada and provincial and territorial governments. The views expressed herein do not necessarily represent the views of Health Canada or any provincial or territorial government. Unless otherwise indicated, this product uses data provided by Canada’s provinces and territories. All rights reserved. The contents of this publication may be reproduced unaltered, in whole or in part and by any means, solely for non-commercial purposes, provided that the Canadian Institute for Health Information is properly and fully acknowledged as the copyright owner. Any reproduction or use of this publication or its contents for any commercial purpose requires the prior written authorization of the Canadian Institute for Health Information. Reproduction or use that suggests endorsement by, or affiliation with, the Canadian Institute for Health Information is prohibited. For permission or information, please contact CIHI: Canadian Institute for Health Information 495 Richmond Road, Suite 600 Ottawa, Ontario K2A 4H6 Phone: 613-241-7860 Fax: 613-241-8120 www.cihi.ca [email protected] © 2018 Canadian Institute for Health Information Cette publication est aussi disponible en français sous le titre Guide de codification des données en obstétrique. Table of contents About CIHI ................................................................................................................................. 6 Chapter 1: Introduction .............................................................................................................. -

Dermatology and Pregnancy* Dermatologia E Gestação*
RevistaN2V80.qxd 06.05.05 11:56 Page 179 179 Artigo de Revisão Dermatologia e gestação* Dermatology and pregnancy* Gilvan Ferreira Alves1 Lucas Souza Carmo Nogueira2 Tatiana Cristina Nogueira Varella3 Resumo: Neste estudo conduz-se uma revisão bibliográfica da literatura sobre dermatologia e gravidez abrangendo o período de 1962 a 2003. O banco de dados do Medline foi consul- tado com referência ao mesmo período. Não se incluiu a colestase intra-hepática da gravidez por não ser ela uma dermatose primária; contudo deve ser feito o diagnóstico diferencial entre suas manifestações na pele e as dermatoses específicas da gravidez. Este apanhado engloba as características clínicas e o prognóstico das alterações fisiológicas da pele durante a gravidez, as dermatoses influenciadas pela gravidez e as dermatoses específi- cas da gravidez. Ao final apresenta-se uma discussão sobre drogas e gravidez Palavras-chave: Dermatologia; Gravidez; Patologia. Abstract: This study is a literature review on dermatology and pregnancy from 1962 to 2003, based on Medline database search. Intrahepatic cholestasis of pregnancy was not included because it is not a primary dermatosis; however, its secondary skin lesions must be differentiated from specific dermatoses of pregnancy. This overview comprises clinical features and prognosis of the physiologic skin changes that occur during pregnancy; dermatoses influenced by pregnancy and the specific dermatoses of pregnancy. A discussion on drugs and pregnancy is presented at the end of this review. Keywords: Dermatology; Pregnancy; Pathology. GRAVIDEZ E PELE INTRODUÇÃO A gravidez representa um período de intensas ções do apetite, náuseas e vômitos, refluxo gastroeso- modificações para a mulher. Praticamente todos os fágico, constipação; e alterações imunológicas varia- sistemas do organismo são afetados, entre eles a pele. -

Postpartum Haemorrhage
Guideline Postpartum Haemorrhage Immediate Actions Call for help and escalate as necessary Initiate fundal massage Focus on maternal resuscitation and identifying cause of bleeding Determine if placenta still in situ Tailor pharmacological management to causation and maternal condition (see orange box). Complete a SAS form when using carboprost. Pharmacological regimen (see flow sheet summary) Administer third stage medicines if not already done so. Administer ergometrine 0.25mg both IM and slow IV (contraindicated in hypertension) IF still bleeding: Administer tranexamic acid- 1g IV in 10mL via syringe driver set at 1mL/minute administered over 10 minutes OR as a slow push over 10 minutes. Administer carboprost 250micrograms (1mL) by deep intramuscular injection Administer loperamide 4mg PO to minimise the side-effect of diarrhoea Administer antiemetic ondansetron 4mg IV, if not already given Prophylaxis Once bleeding is controlled, administer misoprostol 600microg buccal and initiate an infusion of oxytocin 40IU in 1L Hartmann’s at a rate of 250mL/hr for 4 hours. Flow chart for management of PPH Carboprost Bakri Balloon Medicines Guide Ongoing postnatal management after a major PPH 1. Purpose This document outlines the guideline details for managing primary postpartum haemorrhage at the Women’s. Where processes differ between campuses, those that refer to the Sandringham campus are differentiated by pink italic text or have the heading Sandringham campus. For guidance on postnatal observations and care after a major PPH, please refer to the procedure ‘Postpartum Haemorrhage - Immediate and On-going Postnatal Care after Major PPH 2. Definitions Primary postpartum haemorrhage (PPH) is traditionally defined as blood loss greater than or equal to 500 mL, within 24 hours of the birth of a baby (1). -

Ⅰ Obstetric Hemorrhage Safety Bundle Implementation in a Rural
Obstetric Hemorrhage Safety Bundle Implementation in a Rural CAH Dale C Robinson MD,FACOG Chief Ob/Gyn Grande Ronde Hospital La Grande Oregon S Planning for and Responding to Obstetric Hemorrhage California Maternal Quality Care Collaborative Obstetric Hemorrhage Version 2.0 Task Force This project was supported by Title V funds received from the California Department of Public Health; Maternal, Child and Adolescent Health Division 2 CMQCC S California Maternal Quality Care Collaborative S Multidisciplinary Task Force S Recommendations/ Obstetric Safety Bundle S Yellow/Blue Slides Maternal Mortality Rates, Moving Average, California Residents; 1999-2010 16 14 14.0 12.2 13.5 13.2 12 12.7 11.6 12.1 11.5 10 9.4 10.2 8 6 4 Maternal Deaths per 100,000 Live Births 2 0 1999-2001 2000-2002 2001-2003 2002-2004 2003-2005 2004-2006 2005-2007 2006-2008 2007-2009 2008-2010 Three-Year Moving Average SOURCE: State of California, Department of Public Health, California Birth and Death Statistical Master Files, 1999-2010. Maternal mortality for California (deaths ≤ 42 days postpartum) was calculated using ICD-10 cause of death classification (codes A34, O00-O95,O98-O99) for 1999-2010. On average, the mortality rate increased by 2% each year [(95% CI: 1.0%, 4.2%) p=0.06. Poisson regression] for a statistically significant increasing trend from 1999-2010 (p=0.001 one-sided Cochran-Armitage, based on individual year data). Produced by California Department of Public Health, Center for Family Health, Maternal, Child and Adolescent Health Division, December, 2012. North Carolina: Mortality Mostly Preventable Cause of Death (n=108) % of All Deaths % Preventable Cardiomyopathy 21% 22% Hemorrhage 14 93 PIH 10 60 CVA 9 0 Chronic condition 9 89 AFE 7 0 Infection 7 43 Pulmonary embolism 6 17 Berg CJ, Harper MA, Atkinson SM, et al. -

Cord Prolapse
CLINICAL PRACTICE GUIDELINE CORD PROLAPSE CLINICAL PRACTICE GUIDELINE CORD PROLAPSE Institute of Obstetricians and Gynaecologists, Royal College of Physicians of Ireland and the Clinical Strategy and Programmes Division, Health Service Executive Version: 1.0 Publication date: March 2015 Guideline No: 35 Revision date: March 2017 1 CLINICAL PRACTICE GUIDELINE CORD PROLAPSE Table of Contents 1. Revision History ................................................................................ 3 2. Key Recommendations ....................................................................... 3 3. Purpose and Scope ............................................................................ 3 4. Background and Introduction .............................................................. 4 5. Methodology ..................................................................................... 4 6. Clinical Guidelines on Cord Prolapse…… ................................................ 5 7. Hospital Equipment and Facilities ....................................................... 11 8. References ...................................................................................... 11 9. Implementation Strategy .................................................................. 14 10. Qualifying Statement ....................................................................... 14 11. Appendices ..................................................................................... 15 2 CLINICAL PRACTICE GUIDELINE CORD PROLAPSE 1. Revision History Version No. -

Placenta Previa; Maternal and Fetal Outcomes in Major Degree of Placenta 1
PLACENTA PREVIA The Professional Medical Journal www.theprofesional.com ORIGINAL PROF-0-600 DOI: 10.29309/TPMJ/2019.26.03.600 PLACENTA PREVIA; MATERNAL AND FETAL OUTCOMES IN MAJOR DEGREE OF PLACENTA 1. MBBS, FCPS (O & G) PREVIA. Senior Registrar Department of Obstetrics/ Gynaecology Bilawal Medical College, Jamshoro. Ifat Baloch1, Naseem Bajari2, Sabrina Talpur3, Abdul Rehman Siyal4, Saima Naz Shaikh5 2. MBBS, (MCPS) Research Associate ABSTRACT… Objectives: To determine the maternal and fetal outcomes in patients presented Department of Research Centre with major degree of placenta previa at tertiary care Hospital. Study Design: Descriptive cases LUMHS, Jamshoro / Hyderabad. 3. MBBS, FCPS (Obstetrics / series study. Setting: Department of Gynaecology and Obstetrics of Liaquat University Hospital Gynaecology) Hyderabad. Period: One year from March 2015 to February 2016. Subject and Methods: All Assistant Professor patients with major degrees of placenta previa were included in study. Following delivery the Department of O & G examination of neonate was carried out thoroughly including congenital abnormalities, weight Liaquat University of Medical and Health Sciences, Jamshoro / of baby and Apgar score. Babies and mothers were examined within postoperative wards till Hyderabad. stitches removal and systematically examined for any postoperative complication. All the data 4. MBBS, DCH. MD (Peads) was entered in the proforma. Results: Total 50 patients with major degrees of placenta previa Associate Professor Department of Paediatric were selected. Majority of the women 40% belonged to the age group of 30-35 years. Most of Liaquat University of Medical and the women 92.0%, were symptomatic and presented with painless vaginal bleeding. Elective Health Sciences, Jamshoro / cesarean section was performed among 20% patients while 80% patients underwent emergency Hyderabad. -

AUC Instructions / ૂચના
AUC PROVISIONAL ANSWER KEY (CBRT) Name of the post Assistant Professor, Obstetrics and Gynaecology, GSS, Class-1 Advertisement No. 83/2020-21 Preliminary Test held on 08-07-2021 Question No. 001 – 200 (Concern Subject) Publish Date 09-07-2021 Last Date to Send Suggestion(s) 16-07-2021 THE LINK FOR ONLINE OBJECTION SYSTEM WILL START FROM 10-07-2021; 04:00 PM ONWARDS Instructions / ૂચના Candidate must ensure compliance to the instructions mentioned below, else objections shall not be considered: - (1) All the suggestion should be submitted through ONLINE OBJECTION SUBMISSION SYSTEM only. Physical submission of suggestions will not be considered. (2) Question wise suggestion to be submitted in the prescribed format (proforma) published on the website / online objection submission system. (3) All suggestions are to be submitted with reference to the Master Question Paper with provisional answer key (Master Question Paper), published herewith on the website / online objection submission system. Objections should be sent referring to the Question, Question No. & options of the Master Question Paper. (4) Suggestions regarding question nos. and options other than provisional answer key (Master Question Paper) shall not be considered. (5) Objections and answers suggested by the candidate should be in compliance with the responses given by him in his answer sheet. Objections shall not be considered, in case, if responses given in the answer sheet /response sheet and submitted suggestions are differed. (6) Objection for each question should be made on separate sheet. Objection for more than one question in single sheet shall not be considered. ઉમેદવાર નીચેની ૂચનાઓું પાલન કરવાની તકદાર રાખવી, અયથા વાંધા- ૂચન ગે કરલ રૂઆતો યાને લેવાશે નહ (1) ઉમેદવાર વાંધાં- ૂચનો ફત ઓનલાઈન ઓશન સબમીશન સીટમ ારા જ સબમીટ કરવાના રહશે.