Abnormal Flow-Mediated Epicardial Vasomotion in Human Coronary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 20 *Lecture Powerpoint the Circulatory System: Blood Vessels and Circulation
Chapter 20 *Lecture PowerPoint The Circulatory System: Blood Vessels and Circulation *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The route taken by the blood after it leaves the heart was a point of much confusion for many centuries – Chinese emperor Huang Ti (2697–2597 BC) believed that blood flowed in a complete circuit around the body and back to the heart – Roman physician Galen (129–c. 199) thought blood flowed back and forth like air; the liver created blood out of nutrients and organs consumed it – English physician William Harvey (1578–1657) did experimentation on circulation in snakes; birth of experimental physiology – After microscope was invented, blood and capillaries were discovered by van Leeuwenhoek and Malpighi 20-2 General Anatomy of the Blood Vessels • Expected Learning Outcomes – Describe the structure of a blood vessel. – Describe the different types of arteries, capillaries, and veins. – Trace the general route usually taken by the blood from the heart and back again. – Describe some variations on this route. 20-3 General Anatomy of the Blood Vessels Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Capillaries Artery: Tunica interna Tunica media Tunica externa Nerve Vein Figure 20.1a (a) 1 mm © The McGraw-Hill Companies, Inc./Dennis Strete, photographer • Arteries carry blood away from heart • Veins -

Young Adults. Look for ST Elevation, Tall QRS Voltage, "Fishhook" Deformity at the J Point, and Prominent T Waves
EKG Abnormalities I. Early repolarization abnormality: A. A normal variant. Early repolarization is most often seen in healthy young adults. Look for ST elevation, tall QRS voltage, "fishhook" deformity at the J point, and prominent T waves. ST segment elevation is maximal in leads with tallest R waves. Note high take off of the ST segment in leads V4-6; the ST elevation in V2-3 is generally seen in most normal ECG's; the ST elevation in V2- 6 is concave upwards, another characteristic of this normal variant. Characteristics’ of early repolarization • notching or slurring of the terminal portion of the QRS wave • symmetric concordant T waves of large amplitude • relative temporal stability • most commonly presents in the precordial leads but often associated with it is less pronounced ST segment elevation in the limb leads To differentiate from anterior MI • the initial part of the ST segment is usually flat or convex upward in AMI • reciprocal ST depression may be present in AMI but not in early repolarization • ST segments in early repolarization are usually <2 mm (but have been reported up to 4 mm) To differentiate from pericarditis • the ST changes are more widespread in pericarditis • the T wave is normal in pericarditis • the ratio of the degree of ST elevation (measured using the PR segment as the baseline) to the height of the T wave is greater than 0.25 in V6 in pericarditis. 1 II. Acute Pericarditis: Stage 1 Pericarditis Changes A. Timing 1. Onset: Day 2-3 2. Duration: Up to 2 weeks B. Findings 1. -

Basic Rhythm Recognition
Electrocardiographic Interpretation Basic Rhythm Recognition William Brady, MD Department of Emergency Medicine Cardiac Rhythms Anatomy of a Rhythm Strip A Review of the Electrical System Intrinsic Pacemakers Cells These cells have property known as “Automaticity”— means they can spontaneously depolarize. Sinus Node Primary pacemaker Fires at a rate of 60-100 bpm AV Junction Fires at a rate of 40-60 bpm Ventricular (Purkinje Fibers) Less than 40 bpm What’s Normal P Wave Atrial Depolarization PR Interval (Normal 0.12-0.20) Beginning of the P to onset of QRS QRS Ventricular Depolarization QRS Interval (Normal <0.10) Period (or length of time) it takes for the ventricles to depolarize The Key to Success… …A systematic approach! Rate Rhythm P Waves PR Interval P and QRS Correlation QRS Rate Pacemaker A rather ill patient……… Very apparent inferolateral STEMI……with less apparent complete heart block RATE . Fast vs Slow . QRS Width Narrow QRS Wide QRS Narrow QRS Wide QRS Tachycardia Tachycardia Bradycardia Bradycardia Regular Irregular Regular Irregular Sinus Brady Idioventricular A-Fib / Flutter Bradycardia w/ BBB Sinus Tach A-Fib VT PVT Junctional 2 AVB / II PSVT A-Flutter SVT aberrant A-Fib 1 AVB 3 AVB A-Flutter MAT 2 AVB / I or II PAT PAT 3 AVB ST PAC / PVC Stability Hypotension / hypoperfusion Altered mental status Chest pain – Coronary ischemic Dyspnea – Pulmonary edema Sinus Rhythm Sinus Rhythm P Wave PR Interval QRS Rate Rhythm Pacemaker Comment . Before . Constant, . Rate 60-100 . Regular . SA Node Upright in each QRS regular . Interval =/< leads I, II, . Look . Interval .12- .10 & III alike .20 Conduction Image reference: Cardionetics/ http://www.cardionetics.com/docs/healthcr/ecg/arrhy/0100_bd.htm Sinus Pause A delay of activation within the atria for a period between 1.7 and 3 seconds A palpitation is likely to be felt by the patient as the sinus beat following the pause may be a heavy beat. -

Basic ECG Interpretation
12/2/2016 Basic Cardiac Anatomy Blood Flow Through the Heart 1. Blood enters right atrium via inferior & superior vena cava 2. Right atrium contracts, sending blood through the tricuspid valve and into the right ventricle 3. Right ventricle contracts, sending blood through the pulmonic valve and to the lungs via the pulmonary artery 4. Re-oxygenated blood is returned to the left atrium via the right and left pulmonary veins 5. Left atrium contracts, sending blood through the mitral valve and into the left ventricle 6. Left ventricle contracts, sending blood through the aortic Septum valve and to the body via the aorta 1 http://commons.wikimedia.org/wiki/File:Diagram_of_the_human_heart 2 _(cropped).svg Fun Fact….. Layers of the Heart Pulmonary Artery – The ONLY artery in the body that carries de-oxygenated blood Pulmonary Vein – The ONLY vein in the body that carries oxygenated blood 3 4 Layers of the Heart Endocardium Lines inner cavities of the heart & covers heart valves (Supplies left ventricle) Continuous with the inner lining of blood vessels Purkinje fibers located here; (electrical conduction system) Myocardium Muscular layer – the pump or workhorse of the heart “Time is Muscle” Epicardium Protective outer layer of heart (Supplies SA node Pericardium in most people) Fluid filled sac surrounding heart 5 6 http://stanfordhospital.org/images/greystone/heartCenter/images/ei_0028.gif 1 12/2/2016 What Makes the Heart Pump? Electrical impulses originating in the right atrium stimulate cardiac muscle contraction Your heart's -

What Is Single Ventricle?
LifeLife withwith aa SingleSingle VentricleVentricle StephenStephen Crumb,Crumb, APNAPN CoordinatorCoordinator CColumbusolumbus OOhiohio AAdultdult CCongenitalongenital HHearteart DiseaseDisease ProgramProgram Biology 101 Q. Your heart has ______ chambers? A. ___3____ Wrong! You need to study!! But you asked how many chambers MY heart has! My heart has only 3 chambers because I have a single ventricle !! ObjectivesObjectives DescribeDescribe thethe congenitalcongenital heartheart defectsdefects thatthat leadlead toto singlesingle ventricleventricle DiscussDiscuss longlong--termterm issuesissues thatthat maymay arisearise inin peoplepeople withwith singlesingle ventricleventricle andand howhow toto treattreat themthem DiscussDiscuss waysways toto staystay healthyhealthy LearnLearn fromfrom eacheach otherother TheThe NormalNormal HeartHeart WhatWhat isis SingleSingle Ventricle?Ventricle? AbnormalAbnormal heartheart structurestructure presentpresent atat birthbirth OnlyOnly oneone functionalfunctional pumpingpumping chamberchamber (ventricle)(ventricle) SeveralSeveral congenitalcongenital heartheart defectsdefects leadlead toto singlesingle ventricle,ventricle, aa featurefeature ofof allall isis cyanosiscyanosis CyanosisCyanosis BlueBlue colorcolor duedue toto lowlow bloodblood oxygenoxygen CausedCaused byby mixingmixing ofof oxygenoxygen--poorpoor (blue)(blue) bloodblood andand oxygenoxygen--richrich (red)(red) bloodblood MixingMixing isis necessarynecessary forfor initialinitial survivalsurvival HypoplasticHypoplastic LeftLeft -

Visualizing Real Tim E Vasomotion in Vivo Using Optical Coherence
A Visualizing real time vasomotion in vivo using Optical Coherence Tom ography Suhir Abuhajar 2012 Lancaster University-Physics Department-Nonlinear Biomedical Physics Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in Physics ProQuest Number: 11003436 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11003436 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 4 8 1 0 6 - 1346 C ontents Declaration v Abstract vi Acknowledgment vii 1 Introduction 1 1.1 Motivation .................................................................................................... 1 1.2 Structure of the th esis ............................................................................. 2 1.3 Physiology of the skin ............................................................................. 5 1.3.1 Functions of the s k i n ................................................................... 7 1.3.2 Structure of human s k i n ............................................................ 9 1.3.3 -
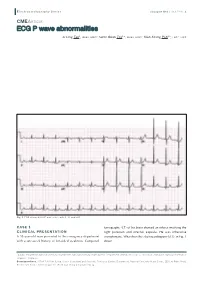
ECG P Wave Abnormalities
E lectrocardiography S eries Singapore Med J 2013; 54(1) : 4 CMEARTICLE ECG P wave abnormalities Li Ling Tan1, MBBS, MRCP, Swee Guan Teo1,2, MBBS, MRCP, Kian Keong Poh1,2, FRCP, FACC Fig. 1 ECG shows bifid P waves in leads II, III and aVF. CASE 1 tomography (CT) of his brain showed an infarct involving the CLINICAL PRESENTATION right putamen and internal capsule. He was otherwise A 58-year-old man presented to the emergency department asymptomatic. What does the electrocardiogram (ECG) in Fig. 1 with a one-week history of left-sided weakness. Computed show? 1Cardiac Department, National University Heart Centre, National University Health System, 2Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore Correspondence: A/Prof Poh Kian Keong, Senior Consultant and Associate Professor, Cardiac Department, National University Heart Centre, 1E Kent Ridge Road, NUHS Tower Block, Level 9, Singapore 119228. [email protected] E lectrocardiography S eries 2a 2b Fig. 2 (a) Echocardiogram in the parasternal long axis view shows a calcified mitral valve. There is restricted opening and closing of the posterior mitral valve leaflet (thick arrow), as well as doming of the anterior mitral valve leaflet (thin arrow); (b) At diastole, the mitral valve opening is markedly reduced (outlined and labeled with an asterisk). RV: right ventricle; LV: left ventricle; LA: left atrium ECG INTERPRETATION CLINICAL COURSE ECG shows the presence of ‘P mitrale’ in multiple leads – inferior Transthoracic echocardiogram showed moderately severe mitral leads II, III and aVF, and also leads V2, V3 and V4. The duration stenosis (Fig. -

Time-Varying Elastance and Left Ventricular Aortic Coupling Keith R
Walley Critical Care (2016) 20:270 DOI 10.1186/s13054-016-1439-6 REVIEW Open Access Left ventricular function: time-varying elastance and left ventricular aortic coupling Keith R. Walley Abstract heart must have special characteristics that allow it to respond appropriately and deliver necessary blood flow Many aspects of left ventricular function are explained and oxygen, even though flow is regulated from outside by considering ventricular pressure–volume characteristics. the heart. Contractility is best measured by the slope, Emax, of the To understand these special cardiac characteristics we end-systolic pressure–volume relationship. Ventricular start with ventricular function curves and show how systole is usefully characterized by a time-varying these curves are generated by underlying ventricular elastance (ΔP/ΔV). An extended area, the pressure– pressure–volume characteristics. Understanding ventricu- volume area, subtended by the ventricular pressure– lar function from a pressure–volume perspective leads to volume loop (useful mechanical work) and the ESPVR consideration of concepts such as time-varying ventricular (energy expended without mechanical work), is linearly elastance and the connection between the work of the related to myocardial oxygen consumption per beat. heart during a cardiac cycle and myocardial oxygen con- For energetically efficient systolic ejection ventricular sumption. Connection of the heart to the arterial circula- elastance should be, and is, matched to aortic elastance. tion is then considered. Diastole and the connection of Without matching, the fraction of energy expended the heart to the venous circulation is considered in an ab- without mechanical work increases and energy is lost breviated form as these relationships, which define how during ejection across the aortic valve. -
Effect of Mental Stress on Myocardial Blood Flow and Vasomotion in Patients with Coronary Artery Disease
Effect of Mental Stress on Myocardial Blood Flow and Vasomotion in Patients with Coronary Artery Disease Heiko Schoder, Daniel H. Silverman, Roxana Campisi, Harold Karpman, Michael E. Phelps, Heinrich R. Schelbert, and Johannes Czernin Department ofMolecular and Medical Pharmacology, Division ofNuclear Medicine, School ofMedicine, University of California, Los Angeles; and Laboratory ofStructural Biology and Molecular Medicine, University ofCalifornia, Los Angeles, California dial oxygen demand is physiologically accommodated by an In patients with coronary artery disease (CAD), mental stress increase in myocardial blood flow (MBF). Therefore, in may provoke ischemic electrocardiographchanges and abnor healthy individuals mental stress induces coronary artery malities in regionaland global left ventricularfunction. However, vasodilatation (3,4). In patients with coronary artery disease little is known about the underlyingmyocardialblood flow (CAD), however, mental stress modeled in a laboratory or response(MBF)inthesepatients.Methods: We investigatedthe experienced in daily life can induce myocardial ischemia as hemodynamic, neurohumoral, and myocardial blood flow re sponses to mental stress in 17 patients with CAD and 17 healthy evidenced by ischemic changes on electrocardiography volunteers of similar age. Mental stress was induced by asking (ECG) (5), left ventricular wall motion abnormalities, and a individualsto solve mathematicsubtractionsin a progressively decline in left ventricular ejection fraction (6, 7). Such challenging sequence; MBF was quantified at rest and during abnormal responses to mental stress may also predict mentalstressusing13NammoniaPET.Results:Mentalstress ischemic events, such as unstable angina pectoris or myocar induced significant (P < 0.01) and comparable increases in dial infarction, in daily life (8,9). rate—pressureproduct, measured in beats per minute x mm Hg, The mechanisms underlying these mental stress—induced in bothpatients(from7826 ±2006to 10586±2800)and healthy volunteers (from 8227 ±1272 to 10618 ±2468). -

Grade 6: the Heart and Circulatory System Lesson 1: the Heart Lesson 2: the Heart Rate Lesson 3: the Circulatory System and Blood
Grade 6: The Heart and Circulatory System Lesson 1: The Heart Lesson 2: The Heart Rate Lesson 3: The Circulatory System and Blood Objectives: 1. Students will identify the four chambers of the heart 2. Students will identify four important structures of the Circulatory System and what they do. 3. Students will explain heart rate and be able to take their resting and active heart rates. 4. Students will describe the major functions of the Circulatory System. 5. Students will explain the role of the heart in circulation 6. Students will give a basic explanation of the cardio-pulmonary sequence. 7. Students will describe systemic circulation. Materials: Lesson 1: • Animal heart (Example: cow, pig, sheep) • Note cards • Picture of the heart (See Figure 1) • Dissection tools (Scissors, pan, etc.) Lesson 2: • Small drum • Watch or clock with second hand, or time • Optional: Stethoscope Lesson 3: • Corn Syrup • Plastic Beads: flat red disks, white ovals, green or blue seed beads • “Explain” experiment (per group): o Two small balloons or large finger cots o One clear tube (1/2” diameter) about 8” long o One clear tube (3/4” diameter) about 8” long o 16 - 20 oz. water o Red food coloring o Measuring cup o Funnel o Two empty plastic containers (such as cottage cheese or yogurt cartons) Activity Summary: In this lesson students will learn the basic functioning of the heart, Circulatory System and blood, the connection to lung functioning, and the activity of the Grade 6: The Heart & Circulatory System – Revised 2008 Page 1 Circulatory System in the body. -

Blood Vessels and Circulation
19 Blood Vessels and Circulation Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Functional Anatomy of Blood Vessels Learning Outcomes 19.1 Distinguish between the pulmonary and systemic circuits, and identify afferent and efferent blood vessels. 19.2 Distinguish among the types of blood vessels on the basis of their structure and function. 19.3 Describe the structures of capillaries and their functions in the exchange of dissolved materials between blood and interstitial fluid. 19.4 Describe the venous system, and indicate the distribution of blood within the cardiovascular system. © 2018 Pearson Education, Inc. Module 19.1: The heart pumps blood, in sequence, through the arteries, capillaries, and veins of the pulmonary and systemic circuits Blood vessels . Blood vessels conduct blood between the heart and peripheral tissues . Arteries (carry blood away from the heart) • Also called efferent vessels . Veins (carry blood to the heart) • Also called afferent vessels . Capillaries (exchange substances between blood and tissues) • Interconnect smallest arteries and smallest veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Two circuits 1. Pulmonary circuit • To and from gas exchange surfaces in the lungs 2. Systemic circuit • To and from rest of body © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits 1. Right atrium (entry chamber) • Collects blood from systemic circuit • To right ventricle to pulmonary circuit 2. Pulmonary circuit • Pulmonary arteries to pulmonary capillaries to pulmonary veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits (continued) 3. Left atrium • Receives blood from pulmonary circuit • To left ventricle to systemic circuit 4. -

The Jugular Venous Pressure Revisited
REVIEW CME EDUCATIONAL OBJECTIVE: Readers will measure and interpret the jugular venous pressure in their patients CREDIT with heart failure JOHN MICHAEL S. CHUA CHIACO, MD NISHA I. PARIKH, MD, MPH DAVID J. FERGUSSON, MD Cardiovascular Disease, John A. Burns School Assistant Professor, John A. Burns School Clinical Professor of Medicine, Department of Medicine, University of Hawaii, Honolulu of Medicine, University of Hawaii; of Cardiology, John A. Burns School The Queen’s Medical Center, Honolulu of Medicine, University of Hawaii; The Queen’s Medical Center, Honolulu The jugular venous pressure revisited ■■ ABSTRACT n this age of technological marvels, I it is easy to become so reliant on them as Assessment of the jugular venous pressure is often inad- to neglect the value of bedside physical signs. equately performed and undervalued. Here, we review Yet these signs provide information that adds the physiologic and anatomic basis for the jugular venous no cost, is immediately available, and can be pressure, including the discrepancy between right atrial repeated at will. and central venous pressures. We also describe the cor- Few physical findings are as useful but as rect method of evaluating this clinical finding and review undervalued as is the estimation of the jugular the clinical relevance of the jugular venous pressure, venous pressure. Unfortunately, many practi- especially its value in assessing the severity and response tioners at many levels of seniority and experi- to treatment of congestive heart failure. Waveforms ence do not measure it correctly, leading to a vicious circle of unreliable information, lack reflective of specific conditions are also discussed.