MMWR, Volume 70, Issue 17 — April 30, 2021
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

12C60-1—Motor Vehicle Commission
Rules of Department of Revenue Division 60–Motor Vehicle Commission Chapter 1–General Rules Title Page 12 CSR 60-1.010 Definitions (Rescinded April 30, 2000) ....................................................3 12 CSR 60-1.020 Missouri Motor Vehicle Commission (Rescinded April 30, 2000) ....................3 12 CSR 60-1.030 General Organization (Rescinded April 30, 2000)........................................3 12 CSR 60-1.040 Policy for Handling Release of Public Records (Rescinded April 30, 2000).........3 12 CSR 60-1.050 Public Complaint Handling and Disposition Procedures (Rescinded April 30, 2000) ..................................................................3 12 CSR 60-1.060 Fees (Rescinded April 30, 2000) ............................................................3 Rebecca McDowell Cook (3/31/00) CODE OF STATE REGULATIONS 1 Secretary of State Chapter 1—General Rules 12 CSR 60-1 Title 12—DEPARTMENT OF 1991, effective Jan. 13, 1992. Amended: REVENUE Filed Nov. 18, 1991, effective April 9, 1992. Division 60—Motor Vehicle Commission Amended: Filed Nov. 21, 1994, effective May Chapter 1—General Rules 28, 1995. Amended: Filed Sept. 19, 1995, effective March 30, 1996. Rescinded: Filed 12 CSR 60-1.010 Definitions Oct. 15, 1999, effective April 30, 2000. (Rescinded April 30, 2000) AUTHORITY: section 301.553, RSMo Supp. 1990. Original rule filed July 10, 1989, effec- tive Sept. 28, 1989. Rescinded: Filed Oct. 15, 1999, effective April 30, 2000. 12 CSR 60-1.020 Missouri Motor Vehicle Commission (Rescinded April 30, 2000) AUTHORITY: section 301.553, RSMo Supp. 1990. Original rule filed July 10, 1989, effec- tive Sept. 28, 1989. Rescinded: Filed Oct. 15, 1999, effective April 30, 2000. 12 CSR 60-1.030 General Organization (Rescinded April 30, 2000) AUTHORITY: section 301.553, RSMo Supp. -
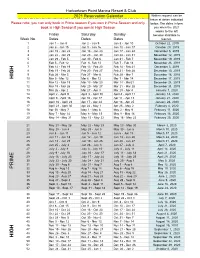
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

9/11 Report”), July 2, 2004, Pp
Final FM.1pp 7/17/04 5:25 PM Page i THE 9/11 COMMISSION REPORT Final FM.1pp 7/17/04 5:25 PM Page v CONTENTS List of Illustrations and Tables ix Member List xi Staff List xiii–xiv Preface xv 1. “WE HAVE SOME PLANES” 1 1.1 Inside the Four Flights 1 1.2 Improvising a Homeland Defense 14 1.3 National Crisis Management 35 2. THE FOUNDATION OF THE NEW TERRORISM 47 2.1 A Declaration of War 47 2.2 Bin Ladin’s Appeal in the Islamic World 48 2.3 The Rise of Bin Ladin and al Qaeda (1988–1992) 55 2.4 Building an Organization, Declaring War on the United States (1992–1996) 59 2.5 Al Qaeda’s Renewal in Afghanistan (1996–1998) 63 3. COUNTERTERRORISM EVOLVES 71 3.1 From the Old Terrorism to the New: The First World Trade Center Bombing 71 3.2 Adaptation—and Nonadaptation— ...in the Law Enforcement Community 73 3.3 . and in the Federal Aviation Administration 82 3.4 . and in the Intelligence Community 86 v Final FM.1pp 7/17/04 5:25 PM Page vi 3.5 . and in the State Department and the Defense Department 93 3.6 . and in the White House 98 3.7 . and in the Congress 102 4. RESPONSES TO AL QAEDA’S INITIAL ASSAULTS 108 4.1 Before the Bombings in Kenya and Tanzania 108 4.2 Crisis:August 1998 115 4.3 Diplomacy 121 4.4 Covert Action 126 4.5 Searching for Fresh Options 134 5. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

April 30, 2021
April 30, 2021 The purpose of publishing aggregated statistical COVID-19 data through the OSDH Dashboard, the Executive Order Report, and the Weekly Epidemiology and Surveillance Report is to support the needs of the general public in receiving important and necessary information regarding the state of the health and safety of the citizens of Oklahoma. These resources may be used only for statistical purposes and may not be used in any way that would determine the identity of any reported cases. Data shown are preliminary and subject to change as additional information is obtained. COVID-19 REPORT Individual Lab Statistics COVID-19 Status Data shown are preliminary and subject to change as additional information is obtained. Information provided through the OSDH Acute Disease Service’s daily surveillance report. Cases Newly Reported Cases1 374 Previous Cases 447,931 Total Cases 448,305 (Active Cases) (10,363) 1 94% of specimens were collected April 15– April 28, 2021 Deaths Newly Reported Deaths 2 72 Previous Deaths (April 20, 2021) 6,716 Total Deaths (April 27, 2021) 6,788 2 Week over week change. Recovered 3 Newly Recovered 257 Previous Recovered 430,897 Total Recovered 431,154 3 Recovered: Currently not hospitalized or deceased and 14 days after onset/report. Status as of midnight the date prior to this report. Oklahoma State Department of Health | Executive Order COVID-19 Report | April 30, 2021 | Page 1 COVID-19 REPORT Individual Lab Statistics Testing and Hospital Status Survey Information provided through survey of Oklahoma hospitals and laboratories as reported to OSDH as of the time of this report. -

2021 HR Pay Calendar Chart
2021 HR Pay Calendar Chart Pay Period Ending Deadline to Have Actions to HR by Noon Pay Day (Check Date) January 15 (current) Friday, January 15, 2021 Wednesday, December 9, 2020 January 29 (lag) January 29 (current) Sunday, January 31, 2021 Wednesday, January 6, 2021 February 12 (lag) February 12 (current) Monday, February 15, 2021 Wednesday, January 20, 2021 February 26 (lag) February 26 (current) Sunday, February 28, 2021 Friday, February 5, 2021 March 15 (lag) March 15 (current) Monday, March 15, 2021 Monday, February 22, 2021 March 31 (lag) March 31 (current) Wednesday, March 31, 2021 Wednesday, March 10, 2021 April 15 (lag) April 15 (current) Thursday, April 15, 2021 Wednesday, March 24, 2021 April 30 (lag) April 30 (current) Friday, April 30, 2021 Thursday, April 8, 2021 May 14 (lag) May 14 (current) Saturday, May 15, 2021 Wednesday, April 21, 2021 May 28 (lag) May 28 (current) Monday, May 31, 2021 Thursday, May 6, 2021 June 15 (lag) June 15 (current) Tuesday, June 15, 2021 Monday, May 24, 2021 June 30 (lag) June 30 (current) Wednesday, June 30, 2021 Tuesday, June 8, 2021 July 15 (lag) July 15 (current) Thursday, July 15, 2021 Wednesday, June 23, 2021 July 30 (lag) July 30 (current) Saturday, July 31, 2021 Thursday, July 8, 2021 August 13 (lag) August 13 (current) Sunday, August 15, 2021 Friday, July 16, 2021 August 31 (lag) August 31 (current) Tuesday, August 31, 2021 Monday, August 2, 2021 September 15 (lag) September 15 (current) Wednesday, September 15, 2021 Tuesday, August 17, 2021 September 30 (lag) September 30 (current) -
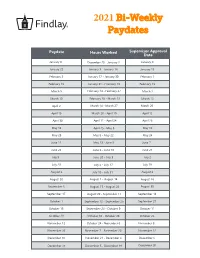
2021 Bi-Weekly Paydates
2021 Bi-Weekly Paydates Paydate Hours Worked Supervisor Approval Date January 8 December 20 - January 2 January 4 January 22 January 3 - January 16 January 15 February 5 January 17 - January 30 February 1 February 19 January 31 - February 13 February 15 March 5 February 14 - February 27 March 1 March 19 February 28 - March 13 March 15 April 2 March 14 - March 27 March 29 April 16 March 28 - April 10 April 12 April 30 April 11 - April 24 April 26 May 14 April 25 - May 8 May 10 May 28 May 9 - May 22 May 24 June 11 May 23 - June 5 June 7 June 25 June 6 - June 19 June 21 July 9 June 20 - July 3 July 2 July 23 July 4 - July 17 July 19 August 6 July 18 - July 31 August 2 August 20 August 1 - August 14 August 16 September 3 August 15 - August 28 August 30 September 17 August 29 - September 11 September 13 October 1 September 12 - September 25 September 27 October 15 September 26 - October 9 October 11 October 29 October 10 - October 23 October 25 November 12 October 24 - November 6 November 8 November 26 November 7 - November 20 November 22 December 10 November 21 - December 4 December 6 December 24 December 5 - December 18 December 20 2021 Bi-Weekly Paydate/Holiday Paydates January February March April Sun Mon Tues Wed Thurs Fri Sat Sun Mon Tues Wed Thurs Fri Sat Sun Mon Tues Wed Thurs Fri Sat Sun Mon Tues Wed Thurs Fri Sat 1 2 1 2 3 4 5 6 1 2 3 4 5 6 1 2 3 4 3 5 6 7 8 9 7 8 9 10 11 12 13 7 8 9 10 11 12 13 4 5 6 7 8 9 10 10 11 12 13 14 15 16 14 15 16 17 18 19 20 14 15 16 17 18 19 20 11 12 13 14 15 16 17 17 18 19 20 21 22 23 21 22 23 24 25 26 -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

Commissioner's Agenda
Commissioner’s Agenda 2021 2020 2019 2018 2017 2021-January-11 2020-January-13 2019-January-14 2018-January-03 2017-January 09 2021-January-27 2020-January-17 2019-January-30 Special Meeting 2017-January-25 2021-February-08 Special Meeting 2019-February-11 2018-January-08 2017-February-13 2021-February-24 2020-January-29 2019-February-27 2018-January-31 2017-February-22 2021-March-08 2020-February-10 2019-March-11 2018-February-12 2017-March-13 2021-March-31 2019-February-26 2019-March-27 2018-February-28 2017-March-29 2021-April-07 2020-March-09 2019-April-08 2018-March-12 2017-April-10 2021-April-12 2020-March-16 2019-April-16 2018-March-28 2017-April-26 2021-April-28 Special Meeting Special Meeting 2018-April-09 2017-May-08 2021-May-05 2020-March-25 2019-April-30 2018-April-25 2017-May-31 Special Meeting 2020-April-13 2019-May-13 2018-May-14 2017-June-12 2021-May-10 2020-April-29 2019-May-29 2018-May-30 2017-June-26 2021-May-26 2020-May-11 2019-June-10 2018-June-11 2017-July-10 2021-June14 2020-May-27 2019-June-24 2018-June-25 2017-July-26 2020-June-08 2019-July-08 2018-July-09 2017-August-14 2020-June-22 2019-July-31 2018-July-25 2017-August-30 2020-July-07 2019-August-02 2018-August-13 2017-September-11 Special Meeting 2019-August-12 2018-August-29 2017-September-29 2020-July-13 2019-August-28 2018-September-10 2017-October-10 2020-July-29 2019-August-28 2018-October-09 2107-October-25 2020-August-10 September Meeting 2018-October-31 2017-November-13 2020-August-26 Date Change Notice 2018-November-13 2017-November-27 2020-September-14 -
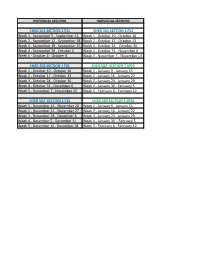
CHED 501-SECTION 1 F21 CHED 501 SECTION 2 F21 Week 1
INDIVIDUAL SESSIONS INDIVIDUAL SESSIONS CHED 501-SECTION 1 F21 CHED 501 SECTION 2 F21 Week 1 - September 5 - September 11 Week 1 - October 10 - October 16 Week 2 - September 12 - September 18 Week 2 - October 17 - October 23 Week 3 - September 19 - September 25 Week 3 - October 24 - October 30 Week 4 - September 26 - October 2 Week 4 - October 31 - November 6 Week 5 - October 3 - October 9 Week 5 - November 7 - November 13 CHED 502 SECTION 1 F21 CHED 502 -SECTION 1 SP22 Week 1 - October 10 - October 16 Week 1 - January 9 - January 15 Week 2 - October 17 - October 23 Week 2 - January 16 - January 22 Week 3 - October 24 - October 30 Week 3 - January 23 - January 29 Week 4 - October 31 - November 6 Week 4 - January 30 - February 5 Week 5 - November 7 - November 13 Week 5 - February 6 - February 12 CHED 503 SECTION 1 F21 CHED 503 SECTION 1 SP22 Week 1 - November 14 - November 20 Week 1 - January 9 - January 15 Week 2 - November 21 - November 27 Week 2 - January 16 - January 22 Week 3 - November 28 - December 4 Week 3 - January 23 - January 29 Week 4 - December 5 - December 11 Week 4 - January 30 - February 5 Week 5 - December 12 - December 18 Week 5 - February 6 - February 12 INDIVIDUAL SESSIONS INDIVIDUAL SESSIONS INDIVIDUAL SESSIONS CHED 501 SECTION 1 SP22 CHED 501 SECTION 2 SP22 Week 1 - January 9 - Janaury 15 Week 1 - April 3 - April 9 Week 2 - January 16 - January 22 Week 2 - April 10 - April 16 Week 3 - January 23 - January 29 Week 3 - April 17 - April 23 Week 4 - January 30 - February 5 Week 4 - April 24 - April 30 Week 5 - February