Kinesin and Myosin: Molecular Motors with Similar Engines Ivan Rayment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mechanical Design of Translocating Motor Proteins
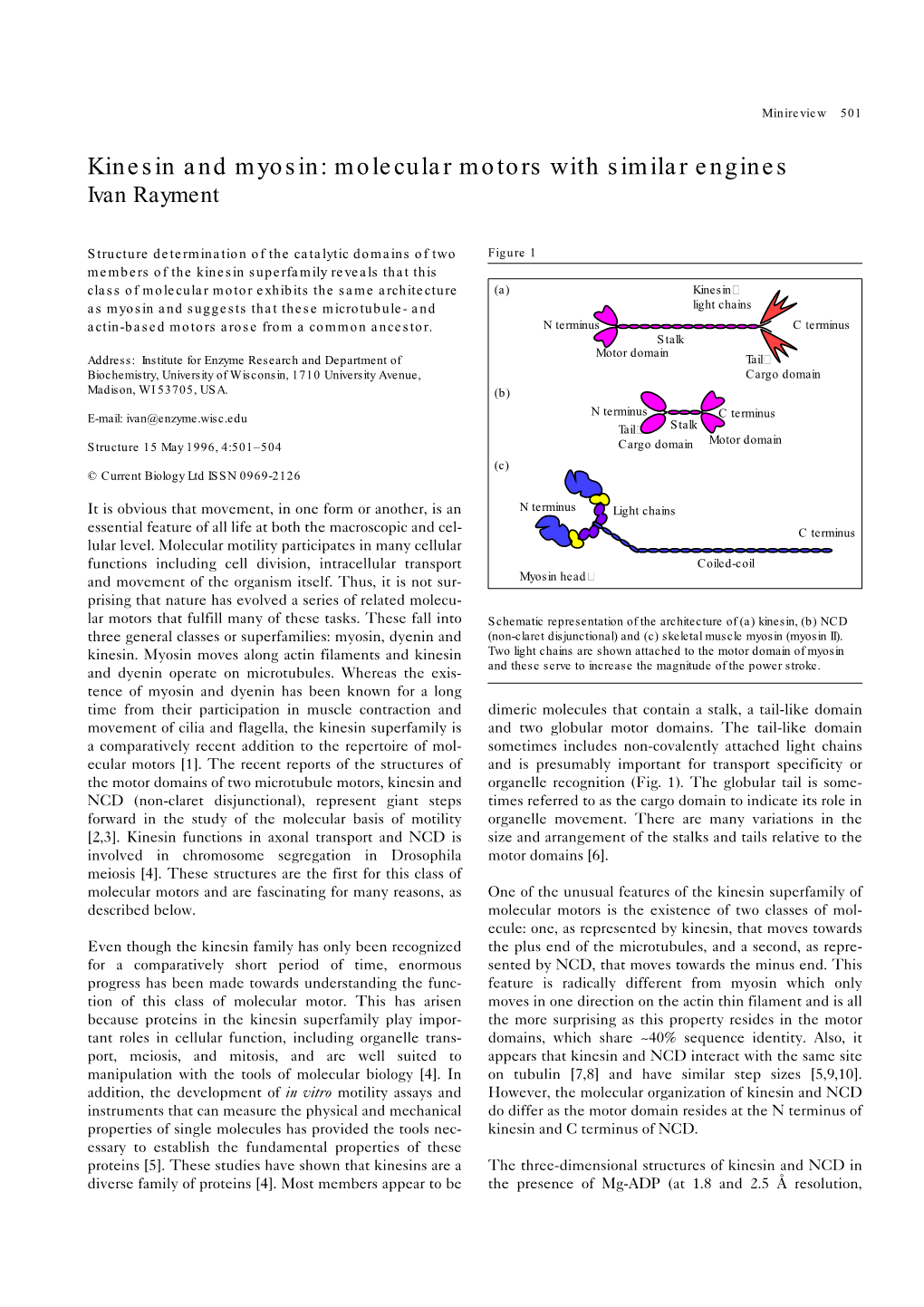
Cell Biochem Biophys (2009) 54:11–22 DOI 10.1007/s12013-009-9049-4 REVIEW PAPER Mechanical Design of Translocating Motor Proteins Wonmuk Hwang Æ Matthew J. Lang Published online: 19 May 2009 Ó Humana Press Inc. 2009 Abstract Translocating motors generate force and move Introduction along a biofilament track to achieve diverse functions including gene transcription, translation, intracellular cargo Motor proteins form distinct classes in the protein universe transport, protein degradation, and muscle contraction. as they can convert chemical energy directly into mechan- Advances in single molecule manipulation experiments, ical work. Among them, translocating motors move along structural biology, and computational analysis are making biopolymer tracks, such as nucleic acids, polypeptides, or it possible to consider common mechanical design princi- quaternary biofilament structures like F-actin or microtu- ples of these diverse families of motors. Here, we propose a bule (Kolomeisky and Fisher proposed the term ‘‘translo- mechanical parts list that include track, energy conversion case’’ for these motors [41]. However, we prefer to use machinery, and moving parts. Energy is supplied not just ‘‘translocating motor,’’ since translocase refers to mem- by burning of a fuel molecule, but there are other sources brane-bound motors such as SecA, whose function is to or sinks of free energy, by binding and release of a fuel or translocate a protein across the membrane [24]). Movement products, or similarly between the motor and the track. is an essential part of their function. For example, RNA Dynamic conformational changes of the motor domain can polymerase (RNAP) walks along the DNA molecule and be regarded as controlling the flow of free energy to and transcribes the genetic code into RNA [25]. -

Chloroplast Transit Peptides: Structure, Function and Evolution
reviews Chloroplast transit Although the first demonstration of precursor trans- port into chloroplasts was shown over two decades peptides: structure, ago3,4, only now is this area of cell biology becom- ing well understood. Many excellent reviews have been published recently on the evolution of plas- function and tids5, the evolution of organelle genomes6, the mechanism of gene transfer from organelles to the nucleus7 and the mechanism of protein import into evolution chloroplasts8,9. Proteins destined to plastids and other organ- elles share in common the requirement for ‘new’ Barry D. Bruce sequence information to facilitate their correct trafficking within the cell. Although in most cases this information resides in a cleavable, N-terminal sequence often collectively referred to as signal It is thought that two to three thousand different proteins are sequence, the different organelle-targeting se- targeted to the chloroplast, and the ‘transit peptides’ that act as quences have distinct properties and names: ‘signal peptides’ for the endoplasmic reticulum, chloroplast targeting sequences are probably the largest class of ‘presequences’ for the mitochondria and ‘transit peptides’ for chloroplasts and other plastids. This targeting sequences in plants. At a primary structural level, transit review focuses on recent progress in dissecting peptide sequences are highly divergent in length, composition and the role of the stromal-targeting domain of chloro- plast transit peptides. I will consider briefly the organization. An emerging concept suggests that transit peptides multitude of distinct functions that transit peptides contain multiple domains that provide either distinct or overlapping perform, provide an update on the limited struc- tural information of a number of transit peptides functions. -

The Wiskott-Aldrich Syndrome: the Actin Cytoskeleton and Immune Cell Function
Disease Markers 29 (2010) 157–175 157 DOI 10.3233/DMA-2010-0735 IOS Press The Wiskott-Aldrich syndrome: The actin cytoskeleton and immune cell function Michael P. Blundella, Austen Wortha,b, Gerben Boumaa and Adrian J. Thrashera,b,∗ aMolecular Immunology Unit, UCL Institute of Child Health, London, UK bDepartment of Immunology, Great Ormond Street Hospital NHS Trust, Great Ormond Street, London, UK Abstract. Wiskott-Aldrich syndrome (WAS) is a rare X-linked recessive primary immunodeficiency characterised by immune dysregulation, microthrombocytopaenia, eczema and lymphoid malignancies. Mutations in the WAS gene can lead to distinct syndrome variations which largely, although not exclusively, depend upon the mutation. Premature termination and deletions abrogate Wiskott-Aldrich syndrome protein (WASp) expression and lead to severe disease (WAS). Missense mutations usually result in reduced protein expression and the phenotypically milder X-linked thrombocytopenia (XLT) or attenuated WAS [1–3]. More recently however novel activating mutations have been described that give rise to X-linked neutropenia (XLN), a third syndrome defined by neutropenia with variable myelodysplasia [4–6]. WASP is key in transducing signals from the cell surface to the actin cytoskeleton, and a lack of WASp results in cytoskeletal defects that compromise multiple aspects of normal cellular activity including proliferation, phagocytosis, immune synapse formation, adhesion and directed migration. Keywords: Wiskott-Aldrich syndrome, actin polymerization, lymphocytes, -

UCSD MOLECULE PAGES Doi:10.6072/H0.MP.A002549.01 Volume 1, Issue 2, 2012 Copyright UC Press, All Rights Reserved
UCSD MOLECULE PAGES doi:10.6072/H0.MP.A002549.01 Volume 1, Issue 2, 2012 Copyright UC Press, All rights reserved. Review Article Open Access WAVE2 Tadaomi Takenawa1, Shiro Suetsugu2, Daisuke Yamazaki3, Shusaku Kurisu1 WASP family verprolin-homologous protein 2 (WAVE2, also called WASF2) was originally identified by its sequence similarity at the carboxy-terminal VCA (verprolin, cofilin/central, acidic) domain with Wiskott-Aldrich syndrome protein (WASP) and N-WASP (neural WASP). In mammals, WAVE2 is ubiquitously expressed, and its two paralogs, WAVE1 (also called suppressor of cAMP receptor 1, SCAR1) and WAVE3, are predominantly expressed in the brain. The VCA domain of WASP and WAVE family proteins can activate the actin-related protein 2/3 (Arp2/3) complex, a major actin nucleator in cells. Proteins that can activate the Arp2/3 complex are now collectively known as nucleation-promoting factors (NPFs), and the WASP and WAVE families are a founding class of NPFs. The WAVE family has an amino-terminal WAVE homology domain (WHD domain, also called the SCAR homology domain, SHD) followed by the proline-rich region that interacts with various Src-homology 3 (SH3) domain proteins. The VCA domain located at the C-terminus. WAVE2, like WAVE1 and WAVE3, constitutively forms a huge heteropentameric protein complex (the WANP complex), binding through its WHD domain with Abi-1 (or its paralogs, Abi-2 and Abi-3), HSPC300 (also called Brick1), Nap1 (also called Hem-2 and NCKAP1), Sra1 (also called p140Sra1 and CYFIP1; its paralog is PIR121 or CYFIP2). The WANP complex is recruited to the plasma membrane by cooperative action of activated Rac GTPases and acidic phosphoinositides. -

Theory of Cytoskeletal Reorganization During Crosslinker-Mediated Mitotic Spindle Assembly
bioRxiv preprint doi: https://doi.org/10.1101/419135; this version posted March 1, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Theory of cytoskeletal reorganization during crosslinker-mediated mitotic spindle assembly A. R. Lamson, C. J. Edelmaier, M. A. Glaser, and M. D. Betterton Abstract Cells grow, move, and respond to outside stimuli by large-scale cytoskeletal reorganization. A prototypical example of cytoskeletal remodeling is mitotic spindle assembly, during which micro- tubules nucleate, undergo dynamic instability, bundle, and organize into a bipolar spindle. Key mech- anisms of this process include regulated filament polymerization, crosslinking, and motor-protein activity. Remarkably, using passive crosslinkers, fission yeast can assemble a bipolar spindle in the absence of motor proteins. We develop a torque-balance model that describes this reorganization due to dynamic microtubule bundles, spindle-pole bodies, the nuclear envelope, and passive crosslink- ers to predict spindle-assembly dynamics. We compare these results to those obtained with kinetic Monte Carlo-Brownian dynamics simulations, which include crosslinker-binding kinetics and other stochastic effects. Our results show that rapid crosslinker reorganization to microtubule overlaps facilitates crosslinker-driven spindle assembly, a testable prediction for future experiments. Combin- ing these two modeling techniques, we illustrate a general method for studying cytoskeletal network reorganization. 1 bioRxiv preprint doi: https://doi.org/10.1101/419135; this version posted March 1, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. -

Construction and Loss of Bacterial Flagellar Filaments
biomolecules Review Construction and Loss of Bacterial Flagellar Filaments Xiang-Yu Zhuang and Chien-Jung Lo * Department of Physics and Graduate Institute of Biophysics, National Central University, Taoyuan City 32001, Taiwan; [email protected] * Correspondence: [email protected] Received: 31 July 2020; Accepted: 4 November 2020; Published: 9 November 2020 Abstract: The bacterial flagellar filament is an extracellular tubular protein structure that acts as a propeller for bacterial swimming motility. It is connected to the membrane-anchored rotary bacterial flagellar motor through a short hook. The bacterial flagellar filament consists of approximately 20,000 flagellins and can be several micrometers long. In this article, we reviewed the experimental works and models of flagellar filament construction and the recent findings of flagellar filament ejection during the cell cycle. The length-dependent decay of flagellar filament growth data supports the injection-diffusion model. The decay of flagellar growth rate is due to reduced transportation of long-distance diffusion and jamming. However, the filament is not a permeant structure. Several bacterial species actively abandon their flagella under starvation. Flagellum is disassembled when the rod is broken, resulting in an ejection of the filament with a partial rod and hook. The inner membrane component is then diffused on the membrane before further breakdown. These new findings open a new field of bacterial macro-molecule assembly, disassembly, and signal transduction. Keywords: self-assembly; injection-diffusion model; flagellar ejection 1. Introduction Since Antonie van Leeuwenhoek observed animalcules by using his single-lens microscope in the 18th century, we have entered a new era of microbiology. -

Review of Molecular Motors
REVIEWS CYTOSKELETAL MOTORS Moving into the cell: single-molecule studies of molecular motors in complex environments Claudia Veigel*‡ and Christoph F. Schmidt§ Abstract | Much has been learned in the past decades about molecular force generation. Single-molecule techniques, such as atomic force microscopy, single-molecule fluorescence microscopy and optical tweezers, have been key in resolving the mechanisms behind the power strokes, ‘processive’ steps and forces of cytoskeletal motors. However, it remains unclear how single force generators are integrated into composite mechanical machines in cells to generate complex functions such as mitosis, locomotion, intracellular transport or mechanical sensory transduction. Using dynamic single-molecule techniques to track, manipulate and probe cytoskeletal motor proteins will be crucial in providing new insights. Molecular motors are machines that convert free energy, data suggest that during the force-generating confor- mostly obtained from ATP hydrolysis, into mechanical mational change, known as the power stroke, the lever work. The cytoskeletal motor proteins of the myosin and arm of myosins8,11 rotates around its base at the catalytic kinesin families, which interact with actin filaments and domain11–17, which can cause the displacement of bound microtubules, respectively, are the best understood. Less cargo by several nanometres18 (FIG. 1B). In kinesins, the is known about the dynein family of cytoskeletal motors, switching of the neck linker (~13 amino acids connecting which interact with microtubules. Cytoskeletal motors the catalytic core to the cargo-binding stalk domain) from power diverse forms of motility, ranging from the move- an ‘undocked’ state to a state in which it is ‘docked’ to the ment of entire cells (as occurs in muscular contraction catalytic domain, is the equivalent of the myosin power or cell locomotion) to intracellular structural dynamics stroke10. -

Intracellular Transport of Influenza Virus Hemagglutinin to the Apical Surface of Madin-Darby Canine Kidney Cells
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Intracellular Transport of Influenza Virus Hemagglutinin to the Apical Surface of Madin-Darby Canine Kidney Cells ENRIQUE RODRIGUEZ-BOULAN, KEVIN T . PASKIET, PEDRO J. I . SALAS, and ENZO BARD Department of Pathology, Downstate Medical Center, State University of New York, Brooklyn, New York 11203 ABSTRACT The intracellular pathway followed by the influenza virus hemagglutinin (HA) to the apical surface of Madin-Darby canine kidney cells was studied by radioimmunoassay, immunofluorescence, and immunoelectron microscopy. To synchronize the migration, we used a temperature-sensitive mutant of influenza WSN, ts61, which, at the nonpermissive temperature, 39.5°C, exhibits a defect in the HA that prevents its exit from the endoplasmic reticulum . Upon transfer to permissive temperature, 32°C, the HA appeared in the Golgi apparatus after 10 min, and on the apical surface after 30-40 min. In the presence of cycloheximide, the expression was not inhibited, indicating that the is defect is reversible; a wave of HA migrated to the cell surface, where it accumulated with a half time of 60 min . After passage through the Golgi apparatus the HA was detected in a population of smooth vesicles, about twice the size of coated vesicles, located in the apical half of the cytoplasm . These HA-containing vesicles did not react with anti-clathrin antibodies . Monensin (10 'UM) delayed the surface appearance of HA by 2 h, but not the transport to the Golgi apparatus. Incubation at 20°C retarded the migration to the Golgi apparatus by ^-30 min and blocked the surface appearance by acting at a late stage in the intracellular pathway, presumably at the level of the post-Golgi vesicles. -

Appropriate Roles of Cardiac Troponins in Evaluating Patients with Chest Pain
J Am Board Fam Pract: first published as 10.3122/jabfm.12.3.214 on 1 May 1999. Downloaded from MEDICAL PRACTICE Appropriate Roles of Cardiac Troponins in Evaluating Patients With Chest Pain Matthew S. Rice, MD, CPT, Me, USA, and David C. MacDonald, DO, Me, USA Background: Diagnosis of acute myocardial infarction relies upon the clinical history, interpretation of the electrocardiogram, and measurement of serum levels of cardiac enzymes. Newer biochemical markers of myocardial injury, such as cardiac troponin I and cardiac troponin T, are now being used instead of or along with the standard markers, the MB isoenzyme of creatine kinase (CK-MB) and lactate dehydrogenase. Methods: We performed a MEDLINE literature search (1987 to 1997) using the key words "troponin I," "troponin T," and "acute myocardial infarction." We reviewed selected articles related to the diagnostic and prognostic usefulness of these cardiac markers in evaluating patients with suspected myocardial infarction. Results: We found that (1) troponin I is a better cardiac marker than CK-MB for myocardial infarction because it is equally sensitive yet more specific for myocardial injury; (2) troponin T is a relatively poorer cardiac marker than CK-MB because it is less sensitive and less specific for myocardial injury; and (3) both troponin I and troponin T may be used as independent prognosticators of future cardiac events. Conclusions: Troponin I is a sensitive and specific marker for myocardial injury and can be used to predict the likelihood of future cardiac events. It is not much more expensive to measure than CK-MB. Over all, troponin I is a better cardiac marker than CK-MB and should become the preferred cardiac enzyme when evaluating patients with suspected myocardial infarction. -

The Mechanics of Intracellular Transport
Developmental Cell Previews Cutting through the Noise: The Mechanics of Intracellular Transport Samantha Stam1,2 and Margaret L. Gardel2,3,* 1Biophysical Sciences Graduate Program, University of Chicago, Chicago, IL 60637, USA 2James Franck Institute and Institute for Biophysical Dynamics, University of Chicago, Chicago, IL 60637, USA 3Department of Physics, University of Chicago, Chicago, IL 60637, USA *Correspondence: [email protected] http://dx.doi.org/10.1016/j.devcel.2014.08.013 Intracellular transport of organelles and proteins is driven by multiple ATP-dependent processes. Recently in Cell, Guo et al. (2014) developed a technique, force-spectrum microscopy, to measure intracellular forces and demonstrate that large motion of cellular components can be produced by random ATP-dependent fluc- tuations within the cytoplasm. Intracellular transport is crucial to diverse mechanisms, and they overcome the Here, Guo et al. (2014) provide the physiological tasks. The cell employs limitations of diffusive transport in at first measurements to directly charac- multiple mechanisms to meet the de- least two distinct ways. One well-appre- terize these ATP-dependent yet random mands of rapidly transporting cell con- ciated mechanism is that molecular forces within the cytoplasm. The authors tents of varying size over large distances, motor proteins drive directed transport measured the mechanics of the cyto- ranging from microns to up to a meter, to of attached cargo along filament tracks plasm using optical tweezers to apply support specific physiological tasks (Figure 1C, blue and black) (Howard, forces to inert particles microinjected (Figure 1A). In a recent issue of Cell, Guo 2001). Motors transport cargo along into the cytoplasm. -

Control of Molecular Motor Motility in Electrical Devices
Control of Molecular Motor Motility in Electrical Devices Thesis submitted in accordance with the requirements of The University of Liverpool for the degree of Doctor in Philosophy By Laurence Charles Ramsey Department of Electrical Engineering & Electronics April 2014 i Abstract In the last decade there has been increased interest in the study of molecular motors. Motor proteins in particular have gained a large following due to their high efficiency of force generation and the ability to incorporate the motors into linear device designs. Much of the recent research centres on using these protein systems to transport cargo around the surface of a device. The studies carried out in this thesis aim to investigate the use of molecular motors in lab- on-a-chip devices. Two distinct motor protein systems are used to show the viability of utilising these nanoscale machines as a highly specific and controllable method of transporting molecules around the surface of a lab-on-a-chip device. Improved reaction kinetics and increased detection sensitivity are just two advantages that could be achieved if a motor protein system could be incorporated and appropriately controlled within a device such as an immunoassay or microarray technologies. The first study focuses on the motor protein system Kinesin. This highly processive motor is able to propel microtubules across a surface and has shown promise as an in vitro nanoscale transport system. A novel device design is presented where the motility of microtubules is controlled using the combination of a structured surface and a thermoresponsive polymer. Both topographic confinement of the motility and the creation of localised ‘gates’ are used to show a method for the control and guidance of microtubules. -

The Kinesin Walk: a Dynamic Model with Elastically Coupled Heads
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 6775-6779, June 1996 Biophysics The kinesin walk: A dynamic model with elastically coupled heads IMRE DERENYI AND TAMA'S VICSEK Department of Atomic Physics, Eotvos University, Budapest, Puskin u 5-7, 1088 Hungary Communicated by Leo P. Kadanoff, University of Chicago, Chicago, IL, February 26, 1996 (received for review November 28, 1995) ABSTRACT Recently individual two-headed kinesin mol- of the related transport phenomena. These models (12-19), ecules have been studied in in vitro motility assays revealing a except that of Ajdari (20), consider cases in which the internal number of their peculiar transport properties. In this paper degree of freedom of the molecular motors is not taken into we propose a simple and robust model for the kinesin stepping account. The purpose of the present work is to define and process with elastically coupled Brownian heads that show all investigate a dynamic model for the kinesin walk that has an of these properties. The analytic and numerical treatment of internal degree of freedom and results in a full agreement with our model results in a very good fit to the experimental data the experimental data for the range of its parameters allowed and practically has no free parameters. Changing the values by the known estimates for the lower and upper bounds of ofthe parameters in the restricted range allowed by the related these parameters. experimental estimates has almost no effect on the shape of There are two basic classes of possible models for the the curves and results mainly in a variation of the zero load stepping of kinesin (21).