RSC Advances
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Methodologies for Food Fraud
Food Fraud Guide Methodologies for Food Fraud Tips for robust experimental results Executive summary Knowing that food fraud scandals often drive public awareness and regulatory changes, the goal of this paper is to present analytical techniques and experimental methodologies, and introduce multivariate statistics and sample class prediction as it relates to food adulteration. Some approaches such as molecular spectroscopy tend to be less expensive, and a few of these instruments have been miniaturized to the point where they can be field-deployed. Spectroscopic instruments are useful in fingerprinting food because small changes in a sample’s spectral profile can be detected with the latest technology, assuming appropriate data normalization techniques are applied. Similarly, the use of both unit- and high-resolution-based mass spectrometry (MS) can be important in food fraud testing because they can fingerprint food based on the pattern of discrete compounds they detect. While other techniques such as inductively coupled plasma mass spectrometry (ICP/MS) and inductively coupled plasma optical emission spectrometry (ICP/OES) have proven adept at identifying geographic origin based on trace element analysis. Genomic testing can accurately identify fish DNA, even from processed samples. From a methodological perspective, nontargeted approaches have proven effective in fingerprinting samples. The advent of inexpensive computer workstations and statistical software has made it possible to link nontargeted workflows with multivariate statistical analysis to extract useful information from analytical data. Until recently, these approaches have been too expensive or complex for researchers to perform by themselves; instead, the data had been handed over to dedicated statisticians or never fully investigated. -

USP Roundtable for DS Protein Standards
USP Roundtable for DS Protein Standards Hosted on February 07, 2017 USP–U.S., Rockville, MD Discussion Agenda Identification Tests for Proteins from Various Sources Quantitative Determination of Proteins Determination of the Purity of Proteins Limits for Contaminants in Proteins Labelling, Packaging, Storage, and Handling 2 Identification Tests for Proteins from Various Sources Current identification tests for proteins used in industry Comprehensive supplier chain qualification program helps reduce routine ID tests at the manufacturing site. Some manufacturers audit suppliers on a quarterly or annual basis. Typical identification tests: appearance, organoleptic, Kjeldahl, Near Infrared (NIR) for process monitoring and QC release. Amino acid profiling is used on a demand basis by customers. Suggested identification tests for proteins from various sources Manufacturers were aware of advanced tests: electrophoresis, CE, peptide mapping, mass spectrometry, ELISA for plant based proteins. Suggested that amino acid profiling in combination with protein profiling with electrophoresis (SDS PAGE) is feasible and suitable. 3 Quantitative Determination of Proteins from Various Sources Current quantification tests for different sources The standard method for protein quantification in industry is Kjeldahl or combustion (Dumas). NIR is commonly used for protein quantification. Total amino acid (AA) contents is believed to provide accurate protein contents. Suggested quantification tests for protein ingredients and finished products containing proteins from various sources Suggested that Kjeldahl or Dumas is a widely accepted quantification method. Total Amino Acids (AA) can be used as a complementary method to Kjeldahl or Dumas. Total AA methods require further standardization and validation. 4 Determination of the Purity of Proteins from Various Sources Impurities/specific tests for proteins Dairy protein industry routinely test for loss on drying (LOD), ash, fat and lactose. -

Thermodynamics and Reaction Mechanism of Urea Decomposition† Cite This: Phys
PCCP View Article Online PAPER View Journal | View Issue Thermodynamics and reaction mechanism of urea decomposition† Cite this: Phys. Chem. Chem. Phys., 2019, 21,16785 a b b b Steffen Tischer, * Marion Bo¨rnhorst, Jonas Amsler, Gu¨nter Schoch and Olaf Deutschmann ab The selective catalytic reduction technique for automotive applications depends on ammonia production from a urea–water solution via thermolysis and hydrolysis. In this process, undesired liquid and solid by-products are formed in the exhaust pipe. The formation and decomposition of these Received 18th March 2019, by-products have been studied by thermogravimetric analysis and differential scanning calorimetry. Accepted 5th July 2019 A new reaction scheme is proposed that emphasizes the role of thermodynamic equilibrium of the DOI: 10.1039/c9cp01529a reactants in liquid and solid phases. Thermodynamic data for triuret have been refined. The observed phenomenon of liquefaction and re-solidification of biuret in the temperature range of 193–230 1Cis rsc.li/pccp explained by formation of a eutectic mixture with urea. Creative Commons Attribution-NonCommercial 3.0 Unported Licence. 1 Introduction and ammonium ISE (ion-selective electrode) measurements. Concluding from experimental results and literature data, 23 Air pollution by nitrogen oxides from Diesel engines is a major possible reactions including urea and its by-products biuret, problem concerning the environment and society. Therefore, cyanuric acid, ammelide, ammeline and melamine are presented. governments follow the need to regulate emissions by law (e.g., Further, cyanate and cyanurate salts and cyanamide are 715/2007/EG, ‘‘Euro 5 and Euro 6’’).1 The favored method to proposed as possible intermediates of high temperature urea reduce nitrogen oxides is selective catalytic reduction (SCR) decomposition. -

4 Hazard Evaluation of Flame Retardants for Printed Circuit Boards
FLAME RETARDANTS IN PRINTED CIRCUIT BOARDS Chapter 4 FINAL REPORT August 2015 EPA Publication 744-R-15-001 4 Hazard Evaluation of Flame Retardants for Printed Circuit Boards This chapter summarizes the toxicological and environmental hazards of each flame-retardant chemical that was identified for potential functional use in printed circuit boards (PCBs) laminates. Evaluations of chemical formulations may also include associated substances (e.g., starting materials, by-products, and impurities) if their presence is specifically required to allow that alternative to fully function in the assigned role. Otherwise, pure substances were analyzed in this assessment. Users of the alternative assessments should be aware of the purity of the trade product they purchase, as the presence of impurities may alter the hazard of the alternative. Toxicological and environmental endpoints included in the hazard profiles are discussed in Section 4.1 along with the criteria used to evaluate each hazard endpoint. Data sources and the review methodology are described in Section 4.2. The report then offers a detailed description of the utility of physical-chemical properties in understanding hazard in Section 4.3 and the process of evaluating human health and environmental endpoints in Section 4.4 and Section 4.5, respectively. A discussion of the evaluation of endocrine activity is included in Section 4.6. The characteristics of each chemical included in the alternatives assessment are summarized in the comparative hazard summary table in Section 4.8. Lastly, the collected data and hazard profile of each chemical are presented in Section 4.9. 4.1 Toxicological and Environmental Endpoints The assessment of endpoints with the intent to create hazard profiles for a Design for the Environment (DfE) alternatives assessment follows the guidance of the DfE Program Alternatives Assessment Criteria for Hazard Evaluation (U.S. -

United States Patent Office E
Unitede States- Patent- Office 3,845,059E. 1. 2 The reaction of biuret with diethanolamine to form PREPARATION OF N,N'-DIETHANOL3,845,059 PIPERAZINE N,N'-diethanol piperazine can be illustrated as follows: Alvin F. Beale, Jr., Lake Jackson, Tex., assignor to The Dow Chemical Company, Midland, Mich. 2(HOCH)NH -- NH2CONHCONH --> No Drawing. Filed June 19, 1972, Ser. No. 264,704 5 CHO Int, C. C07d51/70 U.S. C. 260-268. SY 8 Claims /N al-woman-mamm CH, CH, ABSTRACT OF THE DISCLOSURE + 2CO. 1 + 8NHat Diethanolamine is reacted with urea or a urea pyrol- lo Y yzate (e.g. biuret, triuret, or cyanuric acid) to form N,N'-diethanol piperazine. The following chart illustrates the balanced stoichiom BACKGROUND OF THE INVENTION la etry for reacting diethanolamine with urea, biuret, triuret, N,N'-diethanol piperazine has been previously prepared and cyanuric acid. Reaction products Moles of Empirical Moles N,N'- Moles of formula of of diethanol Moles Moles (HOCH)NH Name of reactant reactant reactant piperazine of Co2 of NH3 2---------------------- Urea------------------------ CHNO 2 2 4. 2- ---. Biuret---. C2HNO2 2 3 6-- --- Triuret.----- ... C3HNO3 2 3. 6 8 6.----- ... Cyanuric aci - C3H3NO3 2 3. 6 6 by the condensation of piperazine with ethylene chloro- The reaction has been found to be specific for dieth hydrin as reported in J. Am. Chem. Soc., Vol. 55, p. 3823 anolamine since analogous dialkanolamines do not give (1933). The compound has been reported to have phar- corresponding dialkanol-substituted cyclic structures con macological properties as an anesthetic or sedative in 30 taining nitrogens within a carbon ring. -

Asian Journal of Chemistry Asian Journal of Chemistry
Asian Journal of Chemistry; Vol. 27, No. 9 (2015), 3149-3151 ASIAN JOURNAL OF CHEMISTRY http://dx.doi.org/10.14233/ajchem.2015.16726 Synthesis and Antimicrobial Activities of 2-S-Hepta-O-benzoyl lactosyl-1-aryl-5-hepta-O-benzoyl-β-lactosyl-2-isothiobiurets * REENA J. DESHMUKH and SHIRISH P. D ESHMUKH P.G. Department of Chemistry, Shri Shivaji College, Akola-444 001, India *Corresponding author: E-mail: [email protected] Received: 20 April 2014; Accepted: 14 March 2015; Published online: 26 May 2015; AJC-17213 A series of novel 2-S-hepta-O-benzoyl lactosyl-1-aryl-5-hepta-O-benzoyl-β-lactosyl-2-isothiobiurets have been synthesized by the interaction of S-hepta-O-benzoyl lactosyl-1-arylisothiocarbamides and hepta-O-benzoyl-β-D-lactosyl isocyanate. These compounds were screened for their antibacterial and antifungal activities against Escherichia coli, Proteus vulgaris, Salmonella typhimurium, Staphylococcus aureus, Pseudomonas aeruginosa and Aspergillus niger. The newly synthesized compounds have been characterized by analytical and IR, 1H NMR and mass spectral studies. Keywords: Arylisothiocarbamides, Lactosyl thiocyanate, Isothiobiurets. INTRODUCTION an efficient synthetic route to novel lactosyl isothiourea derivatives and their antimicrobial activities are reported. A number of thiourea derivatives have been reported to 1 2 exhibit antibacterial , herbicidal and fungicidal activities. EXPERIMENTAL Sugar thioureas3 has synthetic applications in neoglycocon- jugate synthetic strategies4, including neoglycoproteins5, Melting points determined are uncorrected. IR Spectra glycodendrimers6, glycoclusters7 and pseudooligosaccharides8. were recorded on Perkin-Elmer spectrum RXI FTIR spectro- Thiobiurets (mono and di) are also important derivatives meter. 1H NMR was obtained on Bruker DRX-300 MHz NMR of (thio) urea which may increase the biological activity of Spectrometer. -

Spray Foam Event 2013, Session a Presentations Contents List
Spray Foam Event 2013, Session A Presentations Contents List 1. Introduction to Spray Polyurethane Foam (Click to go to PDF Page 2) 2. OSHA’s Isocyanates National Emphasis Program (Click to go to PDF Page 58) 3. EOLWD On-site Consultation Program (Click to go to PDF Page 76) 4. Essential Resources and Training, American Chemistry Council (Click to go to PDF Page 109) 5. Safe Spray Foam (Click to go to PDF Page 126) 6. EPA Safe Use of Polyurethane Products (Click to go to PDF Page 137) Return to Contents List Introduction to Spray Polyurethane Foam This presentation will provide important background information on SPF, including history, product categories and delivery methods and applications. It will also address chemical concerns and include tips for a quality installation, and briefly cover environmental impacts of the product COPYRIGHTED MATERIALS This presentation is protected by US and International copyright laws. Reproduction, distribution, display and use of any part of this presentation without written permission of the speaker is prohibited. © 2013 Spray Polyurethane Foam Alliance Presentation Content 1. History 2. Product Categories 3. Basic Chemistry 4. Delivery Methods 5. Chemical Concerns 6. Environmental Impact 7. Quality Installation 8. Summary History of SPF in Buildings in construction for 50 years • Late 60’s ‐ Medium Density (agricultural and industrial) • Mid 70’s ‐Roofing ‐ Medium Density (general const.) ‐ Sealants • Mid 90’s ‐ Low Density (residential) Product Category Four general categories of SPF Spray Foam -

Title Synthesis of Melamine from Urea, II Author(S)
Title Synthesis of melamine from urea, II Author(s) Kinoshita, Hideo The Review of Physical Chemistry of Japan (1954), 24(1): 19- Citation 27 Issue Date 1954-09-10 URL http://hdl.handle.net/2433/46705 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University The Review of Physical Chemistry of Japan Vol. 24 No. 1 (1954) SYNTHESIS OF MELAMINE FROM UREA, II BS' HILan 1{IYU$H IT Ai it Introduction It was reportedil that the reaction of yielding melamine from urea begins from 275'G, reaches equi]ibrium within 6 hours at 325`C and there is no considerable change in the quantity and the yield of melamine above 325°C. And it was recognized that the reaction velocity is faster, as the packing ratioisgreater and so the pressure of gas phase is-higher. The yield of melamine was calculated from the following equation and the maximum yield was 99.4b. 6NH,CONH_ _ (NH_CN), + 6NH, + 3C0. (1) Moreover, as the intermediate products of this reaction, biuret, cyanuric acid and the water insoluble were obtained. The nitrogen content of this water insoluble ~cas dis- tributedbetween 45.4 and 55.7%. For the purpose of studying the process of this reaction, the author experimented the following cases, the reaction of urea under the condition of existing excess ammonia, the reaction between cyanuric acid and ammonia, the reaction between the water Insoluble and ammonia, and the reaction between melamine and water. These results are compared with those of the previous paper, and moreover the author makes clear that the water insoluble consists of ammelide and ammeline. -
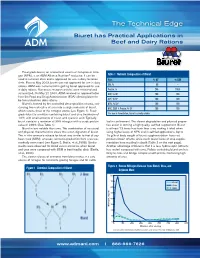
Biuret Applications in Beef and Dairy Rations
The Technical Edge Biuret has Practical Applications in Beef and Dairy Rations Feed grade biuret, an economical source of non-protein nitro- gen (NPN), is an ADM Alliance Nutrition® exclusive. It can be Table 1 Nutrient Composition of Biuret used in ruminant diets and is approved for use in dairy lactation Item % AF % DM diets. Prior to May 2003, biuret was not approved for use in dairy DM, % 99 — rations. ADM was instrumental in getting biuret approved for use in dairy rations. Numerous research articles were reviewed and Protein, % 246 248.5 summarized. On May 27, 2003, ADM received an approval letter RDP, % CP 100 100 from the Food and Drug Administration (FDA) allowing biuret to 1 be formulated into dairy rations. SIP , % CP 100 100 Biuret is formed by the controlled decomposition of urea, con- NPN, % SIP 100 100 densing two molecules of urea into a single molecule of biuret, NRC, 2001 A Protein, % CP 100 100 which retains three of the nitrogen atoms (see Figure 1). Feed- 1 grade biuret is a mixture containing biuret and urea (maximum of For use in formulation; biuret is slowly soluble. 14%) with small amounts of triuret and cyanuric acid. Typically, biuret contains a minimum of 35% nitrogen with a crude protein fed in confinement. The slower degradation and physical proper- value of 246% (See Table 1). ties assist in forming a high-quality, self-fed supplement. Biuret Biuret is less soluble than urea. The combination of structural is at least 7.3 times less toxic than urea, making it ideal when and physical characteristics slows the rumen digestion of biuret. -

Contaminants – Food Compendium
Solutions that meet your demands for food safety testing Excellent choices for food applications Contaminants Acrylamides > Return to Table of Contents > Search entire document Gas Chromatography/Mass Spectrometry Approaches to the Analysis of Acrylamide in Foods Application Food Safety Author Introduction Bernhard Rothweiler The discovery announced in April 2002 by scien- Agilent Technologies tists at Sweden’s National Food Administration of Deutschland GmbH acrylamide (2-propenamide) in fried and baked Hewlett-Packard Strasse 8 foods at levels many times that allowed in water 76337 Waldbronn suggested a much higher exposure than previously Germany estimated [1-3]. Acrylamide (Figure 1), a known neurotoxin, is considered a probable human car- Eberhardt Kuhn cinogen. The World Health Organization considers Agilent Technologies, Inc. 0.5 µg/L the maximum level for acrylamide in 91 Blue Ravine Road water. However, foods such as french fries, baked Folsom, CA potato chips, crisp breads, and other common USA cooked foods, were found to contain acrylamide Harry Prest between 100 and 1000 µg/kg. Acrylamide was not found in the raw foodstuffs and cooking by boiling Agilent Technologies, Inc. produced no detectable levels. Recent work has 5301 Stevens Creek Blvd. suggested that acrylamide forms via the Maillard Santa Clara, CA reaction, which occurs when amino acids and USA sugars (for example, asparagine and sucrose) are heated together [4]. The concern over these rela- Abstract tively high concentrations has led to studies of the occurrence of acrylamide in a wide variety of Discovery of acrylamide in cooked foods has required an foods. examination of foods for potential exposure. A classic H O approach employs extracting acrylamide from the food with water and converting the acrylamide to brominated H H2N derivatives. -

Attachment A
Attachment A Proposed 15-Day Modifications California Code of Regulations, Title 17, Division 3, Chapter 1, Subchapter 7.7, Article 1 Note: This document shows proposed modifications to the originally proposed amendments to the Regulation for the Reporting of Criteria Air Pollutants and Toxic Air Contaminants, as presented during the November 19, 2020, meeting of the California Air Resources Board. At that meeting, the Board directed staff to make modifications to the proposed amendments based on public comments received, and to provide these updates for public comment for a period of at least 15 days. The pre-existing regulation text is set forth below in normal type. The original proposed amendments are shown in underline formatting to indicate additions and strikeout to indicate deletions. The additional proposed modifications made available with the notice of public availability of modified text dated March XY, 2021, are shown in double- underline to indicate additions and double-strikethrough to indicate deletions. The symbol “***” means that intervening text not proposed for amendment is not shown. Proposed Amendments to the Regulation for the Reporting of Criteria Air Pollutants and Toxic Air Contaminants California Code of Regulations, Title 17, Division 3, Chapter 1, Subchapter 7.7, Articles 1 and 2 Amend Subchapter 7.7, Article 1, and sections 93400, 93401, 93402, 93403, 93404, 93405, 93406, 93407, 93408, 93409, 93410, title 17, California Code of Regulations, and adopt new Subchapter 7.7, Article 2, sections 93420, 93421, and new Subchapter 7.7, Article 2, Appendices A and B to title 17, California Code of Regulations, to read as follows: Subchapter 7.7: Regulation for the Reporting of Criteria Air Pollutants and Toxic Air Contaminants Article 1. -

United States Patent Office Patented Feb
3,367,956 United States Patent Office Patented Feb. 6, 1968 1 2 3,367,956 or cycloaliphatic hydrocarbons. When following the pro PREPARATION OF BIURET POLYISOCYANATES cedure of the present invention, it is critical to elevate Hans Joachim Hennig, Cologne-Starnmheim, and Otto the temperature at least in the later stage of the reaction Bayer, Erwin Windemuth, and Wilhelm Bunge, Lever ' to about ISO-250° C. to bring about formation of mono kusen, Germany, assignors to Farhenfabriken Bayer isocyanate which must be continuously removed substan Aktiengesellschaft, Leverkusen, Germany, a German corporation tially as soon as it is formed, preferably by operating at N0 Drawing. Filed Apr. 1, 1964, Ser. No. 356,650 the elevated temperature at a reduced pressure or by Claims priority, application Germany, Apr. 13, 1963, using a carrier gas to remove the organic monoisocyanate F 39,482 from the reaction mixture substantially as soon as it is 6 Claims. (Cl. 260—453) formed. Then the well-de?ned biuret polyisocyanates of the invention are formed instead of high molecular weight This invention relates to organic polyisocyanates and condensation products. Thus, it is an essential feature of more particularly to a process for the preparation of this invention to carry out the process under such tem biuret polyisocyanates. Moreover, this invention provides perature conditions that a monoisocyanate is split off and a new and unexpected way of preparing biuret polyiso the reactants may recombine to form a biuret polyiso cyanates which may also contain carbamyl groups with cyanate. Instead of high molecular weight condensation out the formation of undesirable by-products.