Extraosseus Ewing's Sarcoma in Pancreas: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pediatric Soft Tissue Tumors of Head and Neck – an Update and Review
IP Archives of Cytology and Histopathology Research 2020;5(4):266–273 Content available at: https://www.ipinnovative.com/open-access-journals IP Archives of Cytology and Histopathology Research Journal homepage: https://www.ipinnovative.com/journals/ACHR Review Article Pediatric soft tissue tumors of head and neck – An update and review Shruti Nayak1, Amith Adyanthaya2, Soniya Adyanthaya1,*, Amarnath Shenoy3, M Venkatesan1 1Dept. of Oral Pathology and Microbiology, Yenepoya Dental College, Yenepoya University, Mangalore, Karnataka, India 2Dept. of Pedodontics, KMCT Dental College, Kozhikode, Kerala, India 3Dept. of Conservative and Endodontics, Century Dental College Poinachi, Kasargod, Kerala, India ARTICLEINFO ABSTRACT Article history: Pediatric malignancies especially sarcomas are the most common and predominant cause of mortality in Received 01-12-2020 children. Such ongoing efforts are crucial to better understand the etiology of childhood cancers, get better Accepted 17-12-2020 the survival rate for malignancies with a poor prognosis, and maximize the quality of life for survivors. Available online 30-12-2020 In this review article we authors aim to discuss relatively common benign and malignant connective tissue tumors (soft tissue tumor), focusing on current management strategies and new developments, as they relate to the role of the otolaryngologist– head and neck surgeon. Other rarer paediatric head and neck tumors Keywords: beyond the scope of this review Pediatric Sarcoma © This is an open access article distributed under the terms of the Creative Commons Attribution Soft tissue License (https://creativecommons.org/licenses/by/4.0/) which permits unrestricted use, distribution, and Tumor reproduction in any medium, provided the original author and source are credited. -

Infiltrating Myeloid Cells Drive Osteosarcoma Progression Via GRM4 Regulation of IL23
Published OnlineFirst September 16, 2019; DOI: 10.1158/2159-8290.CD-19-0154 RESEARCH BRIEF Infi ltrating Myeloid Cells Drive Osteosarcoma Progression via GRM4 Regulation of IL23 Maya Kansara 1 , 2 , Kristian Thomson 1 , Puiyi Pang 1 , Aurelie Dutour 3 , Lisa Mirabello 4 , Francine Acher5 , Jean-Philippe Pin 6 , Elizabeth G. Demicco 7 , Juming Yan 8 , Michele W.L. Teng 8 , Mark J. Smyth 9 , and David M. Thomas 1 , 2 ABSTRACT The glutamate metabotropic receptor 4 (GRM4 ) locus is linked to susceptibility to human osteosarcoma, through unknown mechanisms. We show that Grm4 − / − gene– targeted mice demonstrate accelerated radiation-induced tumor development to an extent comparable with Rb1 +/ − mice. GRM4 is expressed in myeloid cells, selectively regulating expression of IL23 and the related cytokine IL12. Osteosarcoma-conditioned media induce myeloid cell Il23 expression in a GRM4-dependent fashion, while suppressing the related cytokine Il12 . Both human and mouse osteosarcomas express an increased IL23:IL12 ratio, whereas higher IL23 expression is associated with worse survival in humans. Con- sistent with an oncogenic role, Il23−/− mice are strikingly resistant to osteosarcoma development. Agonists of GRM4 or a neutralizing antibody to IL23 suppressed osteosarcoma growth in mice. These fi ndings identify a novel, druggable myeloid suppressor pathway linking GRM4 to the proinfl ammatory IL23/IL12 axis. SIGNIFICANCE: Few novel systemic therapies targeting osteosarcoma have emerged in the last four decades. Using insights gained from a genome-wide association study and mouse modeling, we show that GRM4 plays a role in driving osteosarcoma via a non–cell-autonomous mechanism regulating IL23, opening new avenues for therapeutic intervention. -

Problems in Diagnosis Approach for Carcinoma of Pancreatic Head
CASE REPORT Problems in Diagnosis Approach for Carcinoma of Pancreatic Head Ratu Ratih Kusumayanti*, Marcellus Simadibrata**, Murdani Abdullah**, Rino Alvani Gani***, Lies Luthariana* *Department of Internal Medicine, Faculty of Medicine, University of Indonesia Dr. Cipto Mangunkusumo General National Hospital, Jakarta ** Division of Gastroenterology, Department of Internal Medicine, Faculty of Medicine University of Indonesia/Dr. Cipto Mangunkusumo General National Hospital, Jakarta *** Division of Hepatology, Department of Internal Medicine, Faculty of Medicine University of Indonesia/Dr. Cipto Mangunkusumo General National Hospital, Jakarta ABSTRACT Incidences of pancreatic cancer worldwide have been known to be increased. It is the fifth leading cause of death in United State of America. Seventy percent occurs in the head of the pancreas. Major risk factors are related to age, black race, smokers, high-fat diet, chronic pancreatitis, diabetes mellitus and alcohol consumption. Some clinical symptoms such as jaundice, abdominal pain, unexplained weight loss or ascites can occur early or even late in the course of disease. Diagnosing pancreatic cancer sometimes can be difficult, regarding to discrepancy between clinical symptoms and radiological findings. It is important to take good history of the patient, thorough examination, and combine several modalities in diagnosing tumor of pancreatic head. In this case report, a 54 year-old female, came to the hospital with abdominal swelling and jaundice. Physical examination revealed liver and spleen enlargement and edema on both lower extremities. The laboratory result showed increment in Carcinoembryonic Antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) level, without marked increase in bilirubin level. Dilatation of the pancreatic duct was found in this patient, without any sign of bile stone. -

Case Report Metastasis of Pancreatic Cancer Within Primary Colon Cancer by Overtaking the Stromal Microenvironment
Int J Clin Exp Pathol 2018;11(6):3141-3146 www.ijcep.com /ISSN:1936-2625/IJCEP0075771 Case Report Metastasis of pancreatic cancer within primary colon cancer by overtaking the stromal microenvironment Takeo Nakaya1, Hisashi Oshiro1, Takumi Saito2, Yasunaru Sakuma2, Hisanaga Horie2, Naohiro Sata2, Akira Tanaka1 Departments of 1Pathology, 2Surgery, Jichi Medical University, Shimotsuke, Tochigi, Japan Received March 10, 2018; Accepted April 15, 2018; Epub June 1, 2018; Published June 15, 2018 Abstract: We report a unique case of a 74-old man, who presented with double cancers, showing metastasis of pancreatic cancer to colon cancer. Histopathological examination after surgery revealed that the patient had as- cending colon cancer, which metastasized to the liver (pT4N0M1), as well as pancreatic cancer (pT2N1M1) that metastasized to the most invasive portion of the colon cancer, namely the serosal to subserosal layers. Although the mechanisms for this scenario have yet to be elucidated, we speculate that the metastatic pancreatic carcinoma overtook the stromal microenvironment of the colon cancer. Namely, the cancer microenvironment enriched by can- cer-associated fibroblasts, which supported the colon cancer, might be suitable for the invasion and engraftment by pancreatic carcinoma. The similarity of histological appearance might make it difficult to distinguish metastatic pancreatic carcinoma within colon cancer. Furthermore, the metastasis of pancreatic carcinoma in colon carcinoma might be more common, despite it not having been previously reported. Keywords: Cancer metastasis, metastatic pancreatic cancer, colon cancer, double cancer, tumor microenviron- ment Introduction Case report Prevention and control of cancer metastasis is Clinical history one of the most important problems in cancer care [1-4]. -

Primary Ewing Sarcoma/Primitive Neuroectodermal Tumor of the Kidney: the MD Anderson Cancer Center Experience
cancers Article Primary Ewing Sarcoma/Primitive Neuroectodermal Tumor of the Kidney: The MD Anderson Cancer Center Experience Nidale Tarek 1,2,*, Rabih Said 3, Clark R. Andersen 4, Tina S. Suki 1, Jessica Foglesong 1,5 , Cynthia E. Herzog 1, Nizar M. Tannir 6, Shreyaskumar Patel 7, Ravin Ratan 7, Joseph A. Ludwig 7 and Najat C. Daw 1,* 1 Department of Pediatrics, the University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; [email protected] (T.S.S.); [email protected] (J.F.); [email protected] (C.E.H.) 2 Department of Pediatrics and Adolescent Medicine, American University of Beirut Medical Center, Beirut 1107, Lebanon 3 Department of Investigational Cancer Therapeutics, the University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; [email protected] 4 Department of Biostatistics, the University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; [email protected] 5 Division of Hematology, Oncology, Neuro-Oncology & Stem Cell Transplant, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL 60611, USA 6 Department of Genitourinary Medical Oncology, the University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; [email protected] 7 Department of Sarcoma Medical Oncology, the University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; [email protected] (S.P.); [email protected] (R.R.); [email protected] (J.A.L.) * Correspondence: [email protected] (N.T.); [email protected] (N.C.D.); Tel.: +1-713-792-6620 (N.C.D.) Received: 27 August 2020; Accepted: 5 October 2020; Published: 11 October 2020 Simple Summary: The Ewing sarcoma family of tumors (ESFT)s rarely originate in the kidneys and their treatment is significantly different from the other common kidney tumors. -

Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies
Journal of Clinical Medicine Review Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies Richa Rathore 1 and Brian A. Van Tine 1,2,3,* 1 Division of Medical Oncology, Washington University in St. Louis, St. Louis, MO 63110, USA; [email protected] 2 Division of Pediatric Hematology and Oncology, St. Louis Children’s Hospital, St. Louis, MO 63110, USA 3 Siteman Cancer Center, St. Louis, MO 63110, USA * Correspondence: [email protected] Abstract: Osteosarcoma is the most common primary malignant bone tumor in children and young adults. The standard-of-care curative treatment for osteosarcoma utilizes doxorubicin, cisplatin, and high-dose methotrexate, a standard that has not changed in more than 40 years. The development of patient-specific therapies requires an in-depth understanding of the unique genetics and biology of the tumor. Here, we discuss the role of normal bone biology in osteosarcomagenesis, highlighting the factors that drive normal osteoblast production, as well as abnormal osteosarcoma development. We then describe the pathology and current standard of care of osteosarcoma. Given the complex hetero- geneity of osteosarcoma tumors, we explore the development of novel therapeutics for osteosarcoma that encompass a series of molecular targets. This analysis of pathogenic mechanisms will shed light on promising avenues for future therapeutic research in osteosarcoma. Keywords: osteosarcoma; mesenchymal stem cell; osteoblast; sarcoma; methotrexate Citation: Rathore, R.; Van Tine, B.A. Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies. J. Clin. Med. 2021, 1. Introduction 10, 1182. https://doi.org/10.3390/ Osteosarcomas are the most common pediatric and adult bone tumor, with more than jcm10061182 1000 new cases every year in the United States alone. -

Rare Pancreatic Tumors
Published online: 2020-04-29 THIEME 64 ReviewRare Pancreatic Article Tumors Choudhari et al. Rare Pancreatic Tumors Amitkumar Choudhari1,2 Pooja Kembhavi1,2 Mukta Ramadwar3,4 Aparna Katdare1,2 Vasundhara Smriti1,2 Akshay D. Baheti1,2 1Department of Radiodiagnosis, Tata Memorial Hospital, Mumbai, Address for correspondence Akshay D. Baheti, MD, Department of Maharashtra, India Radiodiagnosis, Tata Memorial Hospital, Ernest , Borges Marg Parel 2Department of Radiodiagnosis, Homi Bhabha National University, Mumbai 400012, India (e-mail: [email protected]). Mumbai, Maharashtra, India 3Department of Pathology, Tata Memorial Hospital, Mumbai, Maharashtra, India 4Department of Pathology, Homi Bhabha National University, Mumbai, Maharashtra, India J Gastrointestinal Abdominal Radiol ISGAR 2020;3:64–74 Abstract Pancreatic ductal adenocarcinoma, neuroendocrine tumor, and cystic pancreatic neo- plasms are the common pancreatic tumors most radiologists are familiar with. In this Keywords article we review the clinical presentation, pathophysiology, and radiology of rare pan- ► pancreatic cancer creatic neoplasms. While the imaging features are usually nonspecific and diagnosis is ► uncommon based on pathology, the radiology along with patient demographics, history, and lab- ► pancreatoblastoma oratory parameters can often help indicate the diagnosis of an uncommon pancreatic ► acinar cell neoplasm and guide appropriate management in these cases. ► lymphoma Introduction hyperlipasemia may rarely lead to extraabdominal manifes- tations like ectopic subcutaneous fat necrosis and polyarthri- Pancreatic tumors of various histological subtypes can be tis (lipase hypersecretion syndrome).4 encountered in clinical practice, most common being pan- These tumors are hypoenhancing compared with the pan- creatic ductal adenocarcinoma (PDAC), which constitutes creas and are frequently associated with cystic or necrotic 85% of all pancreatic neoplasms.1 Histologically pancreat- areas as well as calcifications5,6 (►Fig. -
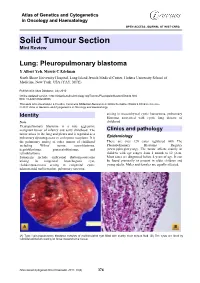
Solid Tumour Section Mini Review
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Solid Tumour Section Mini Review Lung: Pleuropulmonary blastoma Y Albert Yeh, Morris C Edelman North Shore University Hospital, Long Island Jewish Medical Center, Hofstra University School of Medicine, New York, USA (YAY, MCE) Published in Atlas Database: July 2010 Online updated version : http://AtlasGeneticsOncology.org/Tumors/PleuropulmblastomID6040.html DOI: 10.4267/2042/45006 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2011 Atlas of Genetics and Cytogenetics in Oncology and Haematology arising in mesenchymal cystic hamartoma, pulmonary Identity blastoma associated with cystic lung disease of Note childhood. Pleuropulmonary blastoma is a rare aggressive malignant tumor of infancy and early childhood. The Clinics and pathology tumor arises in the lung and pleura and is regarded as a pulmonary dysontogenetic or embryonic neoplasm. It is Epidemiology the pulmonary analog of other tumors of childhood There are over 120 cases registered with The including Wilms' tumor, neuroblastoma, Pleuropulmonary Blastoma Registry hepatoblastoma, pancreatoblastoma, and (www.ppbregistry.org). The tumor affects mainly in retinoblastoma. children with age ranges from 1 month to 12 years. Synonyms include embryonal rhabomyosarcoma Most cases are diagnosed before 4 years of age. It can arising in congenital bronchogenic cyst, be found prenatally or present in older children and rhabdomyosarcoma arising in congenital cystic young adults. Males and females are equally affected. adenomatoid malformation, pulmonary sarcoma (A) Type I pleuropulmonary blastoma consists of multiloculated cyst filled with scanty clear serous fluid. (B) The cysts are lined by cuboidal epithelium resting on loose mesenchymal tissue. -

The Role of Cytogenetics and Molecular Diagnostics in the Diagnosis of Soft-Tissue Tumors Julia a Bridge
Modern Pathology (2014) 27, S80–S97 S80 & 2014 USCAP, Inc All rights reserved 0893-3952/14 $32.00 The role of cytogenetics and molecular diagnostics in the diagnosis of soft-tissue tumors Julia A Bridge Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha, NE, USA Soft-tissue sarcomas are rare, comprising o1% of all cancer diagnoses. Yet the diversity of histological subtypes is impressive with 4100 benign and malignant soft-tissue tumor entities defined. Not infrequently, these neoplasms exhibit overlapping clinicopathologic features posing significant challenges in rendering a definitive diagnosis and optimal therapy. Advances in cytogenetic and molecular science have led to the discovery of genetic events in soft- tissue tumors that have not only enriched our understanding of the underlying biology of these neoplasms but have also proven to be powerful diagnostic adjuncts and/or indicators of molecular targeted therapy. In particular, many soft-tissue tumors are characterized by recurrent chromosomal rearrangements that produce specific gene fusions. For pathologists, identification of these fusions as well as other characteristic mutational alterations aids in precise subclassification. This review will address known recurrent or tumor-specific genetic events in soft-tissue tumors and discuss the molecular approaches commonly used in clinical practice to identify them. Emphasis is placed on the role of molecular pathology in the management of soft-tissue tumors. Familiarity with these genetic events -

Rare Solid Tumors of the Pancreas As Differential Diagnosis of Pancreatic Adenocarcinoma
JOP. J Pancreas (Online) 2012 May 10; 13(3):268-277. ORIGINAL ARTICLE Rare Solid Tumors of the Pancreas as Differential Diagnosis of Pancreatic Adenocarcinoma Sabine Kersting1, Monika S Janot1, Johanna Munding2, Dominique Suelberg1, Andrea Tannapfel2, Ansgar M Chromik1, Waldemar Uhl1, Uwe Bergmann1 Departments of 1General and Visceral Surgery, St. Josef-Hospital, and 2Pathology; Ruhr-University Bochum. Bochum, Germany ABSTRACT Context Rare solid tumors of the pancreas can be misinterpreted as primary pancreatic cancer. Objective The aim of this study was to report our experience in the treatment of patients with rare tumor lesions of the pancreas and to discuss clinical and pathological characteristics in the context of the role of surgery. Design Data from patients of our prospective data-base with rare benign and malignant tumors of the pancreas, treated in our division from January 2004 to August 2010, were analyzed retrospectively. Results One-thousand and ninety-eight patients with solid tumors of the pancreas underwent pancreatic surgery. In 19 patients (10 women, 9 men) with a mean age of 57 years (range: 20-74 years) rare pancreatic tumors (metastasis, solid pseudopapillary tumor, teratoma, hemangioma, accessory spleen, lymphoepithelial cyst, hamartoma, sarcoidosis, yolk sac tumor) were the reason for surgical intervention. Conclusion If rare benign and malignant pancreatic tumors, intrapancreatic metastasis, as well as pancreatic malformations or other abnormalities, present themselves as solid masses of the pancreas, they constitute an important differential diagnosis to primary pancreatic neoplasia, e.g. pancreatic ductal adenocarcinoma. Clinical imaging techniques cannot always rule out malignancy, thus operative exploration often remains the treatment of choice to provide the correct diagnosis and initiate adequate surgical therapy. -

Imaging of Pediatric MSK Tumors
Imaging of Pediatric MSK Tumors Kirsten Ecklund, M.D. Boston Children’s Hospital Harvard Medical School [email protected] Tumor Imaging Goals Diagnosis Treatment Surveillance • Lesion • Size, extent • Local recurrence characterization • Treatment response • Metastatic search • Benign vs malignant – Tissue characterization • DDX (necrosis vs growth) – RECIST guidelines • Extent of disease • Surgical planning – Relationship to neurovascular structures – Measurements for custom reconstruction Current MR Imaging Goals • Highest resolution – even at small FOV • Tissue characterization – Functional imaging – Metabolic imaging • Decrease sedation – Motion correction • Increase acquisition speed Diagnosis: Normal RM Stress fx EWS Leukemia Tumor Mimics/Pitfalls • Inflammatory lesions – Osteoid osteoma – Chondroblastoma – Infection – Myositis ossificans – Histiocytosis – CRMO (CNO) • Trauma/stress fracture 19 y.o. right elbow mass Two 15 year olds with rt knee pain D. Femur stress fx, p. tibia stress reaction Primary bone lymphoma Primary Osseous Lymphoma • 6% of 1° bone tumors, <10% of NHL • Commonly involves epiphyses and equivalents • MR - “Infarct-like” appearance, sequestra • 10-30% multifocal • 10-15% metastases at dx 90% of malignant pediatric bone tumors Osteosarcoma ES family of tumors • ~ 400 new cases/yr in U.S. • ~ 200 new cases/yr • #1 malignant bone tumor < 18 y.o. • Caucasian predominance • Peak age: 13-16 y.o., boys > girls • Peak age: 10-15 y.o • Sites: d. femur (75%), p. tibia, p. • Sites: axial (54%), appendicular -

A Rare Case of Peripheral Primitive Neuroectodermal Tumor in Palate
Surgery and Rehabilitation Case Report ISSN: 2514-5959 A rare case of peripheral primitive neuroectodermal tumor in palate Rajul Ranka1*, Anuj Jain2, Amol Gadbail3 and Minal Chaudhary1 1Oral Pathology and Microbiology, Sharad Pawar Dental College and Hospital, Sawangi(M), Wardha, India 2Department of Trauma and Emergency Medicine, All India Institute of Medical Sciences, Bhopal, India 3Department of Dentistry, Indira Gandhi Government Medical College, Nagpur, India Abstract Primitive Neuroectodermal Tumor (PNET) is an aggressive round cell malignant tumor which belongs to Ewing’s Sarcoma family. Peripheral PNET is rare in head and neck region and even rare in palate with handful of cases reported till date. A case of Peripheral PNET of palate in a 32-year-old female pa tient along with its clinico-pathologic, radiologic and immunohistologic features as well as its management is reported here. We emphasize the need of histopathology and immunohistochemistry (IHC) for early diagnosis of such aggressive tumors to improve the prognosis of patient by providing timely management. Introduction Case report A neuroectodermal tumor is a tumor of the central or peripheral A thirty-two years old female patient of Indian origin reported nervous system. They are divided into two categories viz. Group to the Out-Patient Department of our institute with a complaint of (I) tumors, such as the pituitary adenomas and carcinoid tumors, painful ulcer on her palate since five months. The ulcer was gradually represent tumors that show predominantly epithelial differenti ation. progressive but has now suddenly increased in size since few days. It Group (II) tumors, which incorporate malignant melanoma, olfactory was associated with dull aching, intermittent and localized pain which neuroblastoma, Ewing’s sar coma (EWS) and primitive neuroectodermal aggravated on mastication and relieved with time.