Product Safety Research Policy: What You Need to Know
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Company Profile
Company Profile Founded in 1806, Colgate-Palmolive is a $17.42 billion consumer products company that serves people around the world with well- known brands that make their lives healthier and more enjoyable. Colgate Brands Colgate provides oral care, personal care, home care and pet nutrition products under trusted brands such as: Colgate, Palmolive, Mennen, Softsoap, Irish Spring, Protex, Sorriso, Kolynos, Elmex, Tom’s of Maine, Ajax, Axion, Soupline, Suavitel, Hill’s Science Diet and Hill’s Prescription Diet. Operations Colgate operates in over 75 countries and sells products in over 200 countries and territories. 37,400 Colgate employees drive our success. Headquartered in New York City, Colgate operates through six divisions around the world: ® ® ® North America Latin America Europe/South Pacific Softsoap Blue- PMS 661 Silver- Foil Stamp Africa/Eurasia Asia Hill’s Pet Nutrition Six Divisions The Company has over 50 manufacturing and research facilities globally. The vast majority of Colgate products are manufactured in Colgate-owned facilities. 5 of 5854 Company Profile Business Strength Colgate Values Colgate’s success is linked to the Company’s values of Net Sales ($ millions) Caring, Global Teamwork and Continuous Improvement. 2009 $15,327 2010 $15,564 n 2011 $16,734 Caring 2012 $17,085 The Company cares about people: Colgate people, 2013 $17,420 customers, shareholders and business partners. Colgate is committed to act with compassion, integrity, honesty Dividends Paid(1) (per common share) and high ethics in all situations, to listen with respect 2009 $0.86 to others and to value differences. The Company is 2010 $1.02 also committed to protect the global environment, to 2011 $1.14 enhance the communities where Colgate people live 2012 $1.22 and work, and to be compliant with government laws 2013 $1.33 and regulations. -

Product & Price# of Barcodes
Product & Price# of Barcodes <span class="hilite">Cleaning</span> Air Wick Freshmatic Ultra1 Starter Kit (2 gadgets + 2 refills) - $12.99 Air Wick Freshmatic Ultra4 Refill (4 pack/6.17 oz) - $13.99 Air Wick Scented Oils1 (1 warmer + 7 refills) - $11.99 Air Wick Scented Oils1 Refill (8 pack) - $12.99 Air Wick Candles (twin2 pack) - $9.99 Clorox Foaming Bathroom3 Cleaner (3 pack/30 oz) - $8.49 Clorox Toilet Wand (361 ct refill with bonus wand) - $16.49 Clorox Automatic Toilet1 Bowl Cleaner (6 ct/3.5 oz) - $10.79 Clorox Disinfecting Wipes1 (5 pack/78 ct) - $14.99 Clorox Green Works Natural2 Cleaner (twin pack) - $4.99 Clorox Oxi Magic (7.81 lbs) - $9.99 Clorox Clean Up (1802 oz + 32 oz trigger) - $10.49 Clorox Pool & Spa Shock1 Plus, 12 pk./1 lb. $34.99 Clorox Pool & Spa Algaecide and Clarifier, 2 pk./1 gal. $14.99 Clorox Pump 'n Clean (2 pumps + 2 refills/18 oz) - $9.99 Clorox Bleach (2/182 2oz bottles) - $5.99 Damp Rid Moisture 46 Pack Hanging - $9.99 DampRid Buckets (6 pack)1 - $9.99 Fabuloso All Purpose 3Cleaner (169 oz) - $7.99 Febreze Air Effects (32 pack/9.7 oz) - $6.99 Febreze Air Effects Heavy1 Duty Crisp Clean Air Freshener (twin pack/19.4 oz) - $5.99 Febreze Set and Refresh2 (2 ct) - $7.99 Febreze Fabric 2 ct. 541 oz. - $7.99 Febreze Car Vent Clips3 (4 ct) $8.99 Febreze Unstopables2 (3 pack/8.8 oz) 8.99 Formula 409 (180 oz 1+ 32 oz) - $11.99 Glade PlugIns (2 warmers1 + 6 refills) - $11.49 Glade PlugIns (8 pack2 refill) - $12.99 Kaboom (twin pack/403 oz) - $8.79 Liquid Plumbr Foam Pipesnake2 (3 pack/17 oz) - $8.99 Liquid Plumbr ProStrength3 (twin pack/80 oz) - $9.99 Lysol Neutra Air (3 pack/164 oz) - $9.99 Lysol Neutra Air Sanitizing4 Spray, 3 pk./16 oz. -

COLGATE ULTRA BRITE® ADVANCED WHITENING ALL in ONE CLEAN MINT Drug Facts
COLGATE ULTRA BRITE ADVANCED WHITENING ALL IN ONE CLEAN MINT- sodium fluoride paste, dentifrice Colgate-Palmolive Company Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- COLGATE ULTRA BRITE® ADVANCED WHITENING ALL IN ONE CLEAN MINT Drug Facts Active ingredient Sodium fluoride 0.24% (0.15% w/v fluoride ion) Purpose Anticavity Use helps protect against cavities Warnings Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away. Directions brush teeth thoroughly, preferably after adults and children 2 each meal or at least twice a day, or as years of age and older directed by a dentist or physician children 2 to 6 years use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) children under 2 years ask a dentist or physician Inactive ingredients sorbitol, water, hydrated silica, sodium lauryl sulfate, flavor, PEG-12, tetrasodium pyrophosphate, cocamidopropyl betaine, cellulose gum, sodium saccharin, xanthan gum, titanium dioxide Questions? 1-800-468-6502 Dist. by: COLGATE-PALMOLIVE COMPANY New York, NY 10022 PRINCIPAL DISPLAY PANEL - 170 g Tube Carton CLEAN MINT Ultra brite® ANTICAVITY FLUORIDE TOOTHPASTE WHITENS TEETH Advanced Whitening All in One Toothpaste® VALUE 30% MORE VS. 4.6 OZ SIZE NET WT 6.0 OZ (170 g) COLGATE ULTRA -

Presentation to Analysts
Colgate Palmolive India Ltd …….since 1937 Colgate Palmolive India Limited Slide 1 Disclaimer This Presentation and its related publication, release, webcast or communication (together the “Presentation”) is for general information purposes only. This Presentation does not solicit any action by Colgate-Palmolive (India) Limited (the “Company”) based on the material contained herein. Nothing in this Presentation is intended by the Company to be construed as legal, accounting, investment or tax saving advice. This Presentation includes statements that are, or may be deemed to be, “forward‐looking statements”(except for the historical information). These forward-looking statements appear in several places in the Presentation and are based on certain expectations, assumptions, anticipated developments and various other factors which are not limited to, risk and uncertainties regarding fluctuations in market share, earnings, market growth, intense competition and the pricing environment in the market, consumption level, ability to maintain and manage key business stakeholders and external factors having impact on the conduct of the business not limited to inflation, currency fluctuations, climatic changes, political and regulatory environment, Government policies and changes in international oil prices etc. The Company, therefore, in no way assures, represents, warrants, guaranty, undertake, express or imply, given that such statements, views, projections or forecasts are correct or that the objectives of the Company will be achieved as contained in the Presentation. The Company’s actual results of operations, financial condition, stability, liquidity, and the development of the industry / business sector in which the Company operates, may differ materially from those suggested by the forward‐looking statements contained in this Presentation. -

Cocamidopropyl Betaine
COCAMIDOPROPYL BETAINE Your patch test result indicates that you have a contact allergy to cocamidopropyl betaine. This contact allergy may cause your skin to react when it is exposed to this substance although it may take several days for the symptoms to appear. Typical symptoms include redness, swelling, itching and fluid-filled blisters. Where is cocamidopropyl betaine found? Cocamidopropyl betaine is used in personal care products like shampoos, hand soaps, and toothpastes, and in cosmetics as an emulsifying agent and thickener. It is also used in conditioners to reduce static cling. How can you avoid contact with cocamidopropyl betaine? Avoid products that list any of the following names in the ingredients: • 1-Propanaminium, N-(carboxymethyl)- N,N-dimethyl-3-((1-oxococonut)amino)-, hydroxide, inner salt • N-(2-Aminoethyl)-N-(2-(2- carboxyethoxy)ethyl) beta-alanine, norcoco acyl derivs., disodium salts • N-(Carboxymethyl)-N,N-dimethyl-3-((1- oxococonut)amino)-1-propanam- inium • Cocamidopropyl betaine • Cocamidopropyl dimethyl glycine • CAS RN: 61789-40-0 What are some products that may contain cocamidopropyl betaine? Anti-fungal: Shampoo/Conditioner: • Mycocide NS • Alberto VO5 Extra Body Shampoo • Alberto VO5 Herbal Escapes Moisturizing Shampoo Cosmetics: • Alberto VO5 Moisture Milks Nourishing Shampoo • Gillette Multi-Glide Shave Gel B,CPB • Alberto VO5 Normal Shampoo Hair Coloring Kits: • Aveeno Baby Wash & Shampoo • Clairol Natural Instincts Haircolor, Level 2, Hazelnut-20 • Charles Worthington Big Hair Full Volume Shampoo • -

Colgate Splits Stock, Raises Dividend 17%
Colgate Splits Stock, Raises Dividend 17% New York, New York, March 6, 1997 . The Colgate-Palmolive Board of Directors today increased the quarterly stock dividend 17 percent and declared a two-for-one split of the Company's common stock. Reuben Mark, Chairman and Chief Executive Officer, said: "In 1996, Colgate achieved record sales and earnings, with all geographic divisions around the world contributing to the strong growth. Cash generation was also outstanding, setting an all- time record. Today's action demonstrates our confidence in the continued strong and profitable growth of Colgate's global business. With this latest increase, Colgate's dividend has grown at a compound rate of 16 percent over the past five years, supporting our firm commitment to increase shareholder value." The price of Colgate stock has appreciated 178 percent since the Company's last two-for-one split, on May 15, 1991, compared to a 118 percent climb for the S&P 500 during the same time period. The higher quarterly dividend of $.55 per pre-split share, up from $.47, is to be paid May 15, 1997 to shareholders of record as of April 25, 1997. On an annualized basis, the new rate is $2.20 per share (or $1.10 after the split). Record date for the two-for-one split is the close of business on April 25, 1997, with certificate distribution scheduled for May 15, 1997. As a result of the split, shareholders will receive one additional share of Colgate common stock for each share they hold as of the record date. -

COLGATE Irish Spring Bar Soap
COLGATE-PALMOLIVE Company MATERIAL SAFETY DATA SHEET This industrial Material Safety Data Sheet is not intended for consumers and does not address consumer use of the product. For information regarding consumer applications of this product, refer to the product label. Irish Spring Bar Soap- All Variants Effective Date: February 15, 2007 __________________________________________________________________________________________________ 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION COLGATE-PALMOLIVE COMPANY 300 Park Avenue EMERGENCY TELEPHONE NUMBER: For emergency New York NY 10022 involving spill, leak, fire, exposure or accident, call CHEMTREC (800) 424-9300, day or night PRODUCT NAME: Irish Spring Bar Soap- All Variants CAS NUMBER: Not applicable – product is a mixture For MEDICAL EMERGENCIES involving this product call GENERAL USE: Formulated soap for use in personal (888) 489-3861 hygiene. ___________________________________________________________________________________________________ 2. COMPOSITION/INFORMATION ON INGREDIENTS The following components, present at a concentration of > or = 0.1%, are listed as carcinogens or potential carcinogens OSHA-REGULATED COMPONENTS (present at a by either the National Toxicology Program (NTP), the concentration of > or = 1%): International Agency for Research on Cancer (IARC) or OSHA: Component CAS # % PEL TLV Component CAS # % PEL TLV Not Applicable Titanium IV Dioxide (Anatase) 1317-70-0 0.1 - 0.6 NE 10 mg/m3 __________________________________________________________________________________________________ 3. HAZARDS IDENTIFICATION POTENTIAL HEALTH EFFECTS EYE CONTACT: Causes eye irritation on direct contact. SKIN CONTACT: May cause skin irritation on prolonged or repeated contact. INGESTION: May be harmful if swallowed in large quantities. INHALATION: No adverse effects expected. _________________________________________________________________________________________________ 4. FIRST AID MEASURES EYE CONTACT: Flush eyes with large amounts of water for 15 minutes. Get medical attention if irritation persists. -

Colgate Elects Stephen I. Sadove to Board of Directors
Colgate Elects Stephen I. Sadove To Board of Directors New York, New York, June 7, 2007... Colgate-Palmolive Company (NYSE:CL) announced today that Stephen I. Sadove, Chairman and Chief Executive Officer of Saks, Inc., has been elected to Colgate's Board of Directors. Mr. Sadove, 55, brings extensive business leadership experience and a strong marketing and consumer products background to the Colgate Board. He joined the management team of Saks as Vice Chairman in 2002 and was named Chief Operating Officer in 2004 and CEO in 2006. A member of the Saks Board of Directors since 1998, Mr. Sadove was elected Chairman in May. Mr. Sadove's strong consumer products experience began in 1975 at General Foods USA, where he held various positions of increasing responsibility, including Executive Vice President and General Manager of the Desserts Division. He joined Bristol- Myers Squibb Company in 1991 as President of Clairol in the United States and later gained additional responsibility for the consumer businesses in Canada, Europe, the Middle East, Africa, and Latin America. In 1996, he was named President of Bristol-Myers Squibb's worldwide beauty care business and was later named a senior vice president with additional responsibility for Mead Johnson Nutritionals. Mr. Sadove is a graduate of Hamilton College and holds an MBA with distinction from Harvard Business School. * * * About Colgate-Palmolive: Colgate-Palmolive is a leading global consumer products company, tightly focused on Oral Care, Personal Care, Home Care and Pet Nutrition. Colgate sells its products in over 200 countries and territories around the world under such internationally recognized brand names as Colgate, Palmolive, Mennen, Softsoap, Irish Spring, Protex, Sorriso, Kolynos, Elmex, Tom's of Maine, Ajax, Axion, Soupline, and Suavitel, as well as Hill's Science Diet and Hill's Prescription Diet. -

AVENUE MAGAZINE • AVENUE on the BEACH • FEBRUARY 2015 FEBRUARY 2015 • AVENUE MAGAZINE • AVENUE on the BEACH | 73 T Takes a Certain Character to Become a Sailor
Sailing the High Steve and Doris Colgate on their maritime adventures and Sthe 50th anniversary of easthe Offshore Sailing School. by Mi Mi Chloe Park Steve Colgate sailing Nirvana in front of Sydney harbor during the Hobart Race of 1980 72 | AVENUE MAGAZINE • AVENUE ON THE BEACH • FEBRUARY 2015 FEBRUARY 2015 • AVENUE MAGAZINE • AVENUE ON THE BEACH | 73 t takes a certain character to become a sailor. Aside from the physical toll, there is a mental resilience required, and seasoned sailors will often remark that deep discipline and respect of the high seas is necessary to succeed. Hemingway said, “Man is never lost at sea,” and Steve Colgate is one such man. His interests led him to charter a course far more adventurous than he could have ever imagined. Since its inception 50 years ago, the Offshore Sailing School has become America’s foremost sailing academy. With his wife of 46 years, Doris, the pair own and operate facilities in New York, South Florida and the Caribbean. Growing up, Steve had no interest in running an empire like the one his ancestor William Colgate started in the 19th century. As Doris remembers it, “His godmother was a Tiffany, and she kept telling him to get a real job and get into the stock market and what the others [in his family] were doing. But it was something he’d said over and over again that he just didn’t want to do.” It was quite a legacy. In 1806 William Colgate created the William Colgate Company in New York. An English manufacturer from Kent, he immigrated to the United States and opened a starch, soap and candle factory on Dutch Street in downtown Manhattan. -

IRISH SPRING SOAP BAR ORIGINAL This Industrial Safety Data Sheet Is Not Intended for Consumers and Does Not Address Consumer Use of the Product
NA GHS IRISH SPRING SOAP BAR ORIGINAL This industrial Safety Data Sheet is not intended for consumers and does not address consumer use of the product. For information regarding consumer applications of this product, refer to the product label. Version 1.0 SDS Number: 660000000042 Revision Date: 09/15/2015 SECTION 1. IDENTIFICATION Product name : IRISH SPRING SOAP BAR ORIGINAL Product code : 200000024199 Material : B02408820007 Manufacturer or supplier's details Company : Colgate-Palmolive Co 300 Park Avenue New York, NY 10022 Telephone : US: Consumer Affairs - 1-800-468-6502 Emergency telephone : For emergencies involving spill, leak, fire, exposure or acci- number dent call CHEMTREC (24hr) at (800) 424-9300 or (703) 527-3887. Medical Emergency For MEDICAL EMERGENCIES involving this product call: (24HR): (888) 489-3861 Recommended use of the chemical and restrictions on use Recommended use : Personal care A formulated soap bar SECTION 2. HAZARDS IDENTIFICATION GHS Classification Not a hazardous substance or mixture. GHS Label element Not a hazardous substance or mixture. Precautionary statements : Response: P308 + P313 IF exposed or concerned: Get medical advice/ attention. Disposal: P501 Dispose of contents/ container to an approved waste dis- posal plant. Other hazards None known. SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS Hazardous components 1 / 10 NA GHS IRISH SPRING SOAP BAR ORIGINAL This industrial Safety Data Sheet is not intended for consumers and does not address consumer use of the product. For information regarding consumer applications of this product, refer to the product label. Version 1.0 SDS Number: 660000000042 Revision Date: 09/15/2015 Chemical Name CAS-No. Concentration (% w/w) COCONUT ACID 61788-47-4 >= 1 - < 5 GLYCERIN 56-81-5 >= 1 - < 5 SODIUM CHLORIDE 7647-14-5 >= 1 - < 5 SECTION 4. -
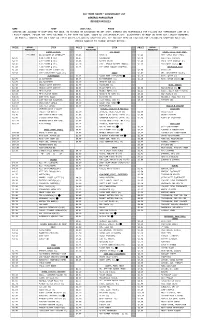
Fcc Terre Haute ~ Commissary List General Population Revised 04/01/13
FCC TERRE HAUTE ~ COMMISSARY LIST GENERAL POPULATION REVISED 04/01/13 NAME__________________________ REG#___________________UNIT/CELL_____________PHASE_______ INMATES ARE ALLOWED TO SHOP ONCE PER WEEK. NO REFUNDS OR EXCHANGES ON ANY ITEM. INMATES ARE RESPONSIBLE FOR FILLING OUT COMMISSARY LIST IN A LEGIBLE MANNER. ENSURE THE ITEM YOU MARK IS THE ITEM YOU WANT. UNDER NO CIRCUMSTANCES WILL ADJUSTMENTS BE MADE ON ITEMS NOT CLEARLY NUMBERED OR MARKED. INMATES MAY ONLY SHOP ON THEIR UNIT’S DESIGNATED SHOPPING DAY. SEE HOLIDAY MEMO ON TRULINCS FOR DESIGNATED SHOPPING ROTATION. PRICES SUBJECT TO CHANGE WITHOUT NOTICE. PRICE AMOUNT ITEM PRICE AMOUNT ITEM PRICE AMOUNT ITEM REQUESTED REQUESTED REQUESTED STAMPS & MISC. ICE CREAM DAIRY, FISH, MEAT CONT. ** LIMIT $9.20 WORTH OF STAMPS!** $1.85 VANILLA $1.05 BEEF STICK JACK LINKS $0.01 0.01 STAMP @ (SL) $1.85 STRAWBERRY $3.40 SWEET SUE CHICKEN $0.17 0.17 STAMP @ (SL) $1.85 BUTTER PECAN $2.05 HALAL BEEF SAUSAGE (h) $0.46 0.46 STAMP @ (SL) $2.10 CHOC. PEANUT BUTTER CHNK(k) $1.95 TACO MEAT (k)(v) $1.00 1.00 STAMP @ (SL) $1.95 ICE CREAM SNDWCH COOKIE(k) MICROWAVE,RICE $1.00 PHOTO TICKET (LIMIT 10) $3.50 PIZZA KIT $7.50 COPY CARD(LIMIT 3)@(L,SL) CANDY $1.05 VEL. MAC&CHEESE (k)(h) ELECTRONICS $2.25 SUGAR FREE LIFESAVERS $1.10 RICE, WHITE (k) $2.05 AA BATTERIES $0.85 BUTTERFINGER (k) $1.10 RICE, BROWN (k)(h) $2.05 AAA BATTERIES $0.80 HERSHEY BAR (k) $2.50 CR2016 WATCH BATTERY $1.30 TWIZZLERS (k) NUTS, SNACKS $2.50 CR2025 WATCH BATTERY $0.85 PLAIN M&M’S (k) $4.30 RAW ALMONDS (k) $69.20 MP-3 PLAYER $0.85 -

Palmolive Bar Soap
COLGATE-PALMOLIVE Company MATERIAL SAFETY DATA SHEET This industrial Material Safety Data Sheet is not intended for consumers and does not address consumer use of the product. For information regarding consumer applications of this product, refer to the product label. Cashmere Bouquet, Irish Spring Soap Bar, Palmolive Soap Bar, Protex Soap Bar, Vel Soap Bar Effective Date: November 2, 2001 __________________________________________________________________________________________________ 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Acute Health…1 Fire…………….1 Reactivity…….0 HMIS HAZARD RATING Least=0 Slight=1 Moderate=2 High=3 Extreme =4 PRODUCT NAME: Cashmere Bouquet, Irish Spring Soap Bar, Palmolive Soap Bar, Protex Soap Bar, Vel Soap Bar COLGATE-PALMOLIVE COMPANY 300 Park Avenue SKU#: Cashmere Bouquet: 12400,12401,12402,12403 New York NY 10022 Irish Spring: 14103,14203,14208,14300,14307,14308, 14321,14326, 14336, 14337,14362,14372,14374,14377, EMERGENCY TELEPHONE NUMBER: For emergency 14378,14402,14435,14803,14807,14826,14874,14878, involving spill, leak, fire, exposure or accident, call 114201 CHEMTREC (800) 424-9300, day or night Palmolive Essential: 19030,18830 Palmolive Original: 10321,10323,10324,10099,323002. THE PITTSBURGH POISON CENTER HAS BEEN 323000,323001,116531,116535,116534,116538 PROVIDED SPECIFIC INFORMATION FOR USE IN Palmolive Naturals: 6531,6532,6534,6535,6536,6537, MEDICAL EMERGENCIES INVOLVING THIS PRODUCT: 6538,6541 CALL COLLECT: (412) 692-5596. Protex Antibacterial Bar Soap: 10802,10700,10702,110705, 110730 Protex