Vegetation Community Monitoring at Fort Sumter National Monument, 2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -
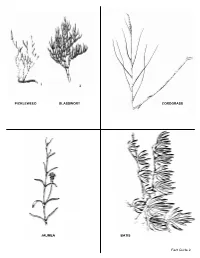
Plant Field Guide
2 PICKLEWEED GLASSWORT CORDGRASS JAUMEA BATIS Field Guide 9 PICKLEWEED Amaranth Family 3 kinds, 2 examples CORDGRASS Grass Family 1 Pickleweed Sarcocornia pacifica Spartina foliosa Glasswort Arthrocnemum subterminalis 2 HABITAT: Growns in the low marsh where the HABITAT: Found throughout the salt marsh. roots are continually bathed in ocean water. APPEARANCE: Stems look like a chain of small APPEARANCE: Look for a tall grass which is pickles. higher than the other plants in the salt marsh. REPRODUCTION: The flowers of all pickleweeds REPRODUCTION: All grasses are wind pollinated. are pollinated by the wind. The small flowers are Look for straw colored spikes of densely packed hard to see because they have no colorful petals flowers. Male flowers will have pollen and the female flowers will show graceful waving stigmas to ADAPTATION TO SALT: Pickleweeds are some of catch the pollen. the many marsh plants that use salt storage (they are accumulators). Also called succulents, these ADAPTATION TO SALT: All the salt marsh plants are swollen with the stored salty water. grasses are salt excreters using special pores to When the salt concentration becomes too high the push out droplets of salty water. Look on the grass cells will die. blades for salt crystals. See sea lavender. ECOLOGICAL RELATIONSHIPS: Frequently the ECOLOGICAL RELATIONSHIPS: Home for the most common plants in the marsh, they provide endangered bird, the Light-footed Clapper Rail. shelter and food for invertebrates. Belding’s A spider lives its whole life inside the blades. Savannah Sparrows build their nests in the Important food for grazing animals. glasswort. BATIS or SALTWORT Saltwort Family Batis maritima HABITAT: Most frequently found in the low marsh. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Plantae, Magnoliophyta, Asterales, Asteraceae, Senecioneae, Pentacalia Desiderabilis and Senecio Macrotis: Distribution Extensions and First Records for Bahia, Brazil
Check List 4(1): 62–64, 2008. ISSN: 1809-127X NOTES ON GEOGRAPHIC DISTRIBUTION Plantae, Magnoliophyta, Asterales, Asteraceae, Senecioneae, Pentacalia desiderabilis and Senecio macrotis: Distribution extensions and first records for Bahia, Brazil. Aristônio M. Teles João R. Stehmann Universidade Federal de Minas Gerais, Instituto de Ciências Biológicas, Departamento de Botânica. Caixa Postal 486, CEP 31270-091, Belo Horizonte, MG, Brazil. E-mail: [email protected] Senecioneae is the biggest Tribe of the Asteraceae state of Minas Gerais (Cabrera 1957; Hind (Nordestam 1996), comprising 150 genera (more 1993a). Senecio macrotis is a robust herb or than 9 % of all genera) and 3,500 species (about shrub, with lyrate-pinnatisect leaves, discoid 15 % of all species of the Family) (Nordenstam heads, and paniculate capitulescences (Cabrera 2007). The circumscription of many Senecioneae 1957). It is found typically in the Campos genera has changed, especially Senecio L., with Rupestres of the Espinhaço range, growing in about 1,250 species (Bremer 1994; Frodin 2004; altitudes ranging from 900 to 1,000 m (Vitta 2002). Nordenstam 2007). To Brazilian Senecioneae, Hind (1993a) estimated the occurrence of 97 The genus Pentacalia Cass., formerly included in species belonging to eight genera, and the more the synonymy of Senecio (lato sensu) (Barkley useful works to identify them are Cabrera (1950, 1985) and resurrected by Robinson and 1957), Cabrera and Klein (1975), Robinson Cuatrecasas (1978), comprises about 205 species (1980), Hind (1993a; 1993b; 1994; 1999), and distributed along Tropical America (Jeffrey 1992). Teles et al. (2006). Hind (1993a) cited the occurrence of two Brazilian species, P. desiderabilis (Vell.) Cuatrec. Senecio (stricto sensu) is characterized by annual and P. -

BOTANY SECTION Compiled by Richard E. Weaver, Jr., Ph.D., and Patti J
TRI-OLOGY, Vol. 47, No. 1 Patti J. Anderson, Ph.D., Managing Editor JANUARY-FEBRUARY 2008 DACS-P-00124 Wayne N. Dixon, Ph. D., Editor Page 1 of 10 BOTANY SECTION Compiled by Richard E. Weaver, Jr., Ph.D., and Patti J. Anderson, Ph.D. For this period, 81 specimens were submitted to the Botany Section for identification, and 795 were received from other sections for identification/name verification, for a total of 876. In addition, 163 specimens were added to the herbarium. Some of the samples received for identification are discussed below: Ageratina jucunda (Greene) Clewell & Woot. (A genus of about 290 species mainly native to the eastern United States and warm regions of the Americas.) Compositae/Asteraceae. Hammock snakeroot. This fall-flowering perennial grows 40–80 cm tall with an erect, minutely pilose stem. The narrowly elliptic to deltoid, 2–6 cm long, opposite leaves are usually glabrous and have crenate to serrate margins. The flower heads contain clusters of white or pinkish-white disc flowers, but no ray flowers. Even without ray flowers, this species provides a stunning display with white clouds of color in the sandhills and hammocks of Georgia and peninsular Florida. Hammock snakeroot, the common name for this species, suggests both its habitat preference for hammocks and the use of members of the genus as a cure for snakebites by indigenous people. (Hillsborough County; B2008-8; Jason B. Sharp; 7 January 2008) (Austin 2004; Mabberley 1997; http://www.efloras.org) Calophyllum inophyllum L. (A genus of 187 tropical species.) Guttiferae/Clusiaceae. Alexandrian laurel, beauty-leaf. -

NATIONAL WETLANDS INVENTORY and the NATIONAL WETLANDS RESEARCH CENTER PROJECT REPORT FOR: GALVESTON BAY INTRODUCTION the U.S. Fi
NATIONAL WETLANDS INVENTORY AND THE NATIONAL WETLANDS RESEARCH CENTER PROJECT REPORT FOR: GALVESTON BAY INTRODUCTION The U.S. Fish & Wildlife Service's National Wetlands Inventory is producing maps showing the location and classification of wetlands and deepwater habitats of the United States. The Classification of Wetlands and Deepwater Habitats of the United States by Cowardin et al. is the classification system used to define and classify wetlands. Upland classification will utilize the system put forth in., A Land Use and Land Cover Classification System For Use With Remote Sensor Data. by James R. Anderson, Ernest E. Hardy, John T. Roach, and Richard E. Witmer. Photo interpretation conventions, hydric soils-lists and wetland plants lists are also available to enhance the use and application of the classification system. The purpose of the report to users is threefold: (1) to provide localized information regarding the production of NWI maps, including field reconnaissance with a discussion of imagery and interpretation; (2) to provide a descriptive crosswalk from wetland codes on the map to common names and representative plant species; and (3) to explain local geography, climate, and wetland communities. II. FIELD RECONNAISSANCE Field reconnaissance of the work area is an integral part for the accurate interpretation of aerial photography. Photographic signatures are compared to the wetland's appearance in the field by observing vegetation, soil and topography. Thus information is weighted for seasonality and conditions existing at the time of photography and at ground truthing. Project Area The project area is located in the southeastern portion of Texas along the coast. Ground truthing covered specific quadrangles of each 1:100,000 including Houston NE, Houston SE, Houston NW, and Houston SW (See Appendix A, Locator Map). -

H. F. Gutiérrez Y O. Morrone - Novedades Nomenclaturalesissn En 0373-580Cenchrus X Bol
Bol. Soc. Argent. Bot. 47 (1-2) 2012 H. F. Gutiérrez y O. Morrone - Novedades nomenclaturalesISSN en 0373-580Cenchrus X Bol. Soc. Argent. Bot. 47 (1-2): 263-269. 2012 NOVEDADES NOMENCLATURALES EN CENCHRUS S.L. (POACEAE: PANICOIDEAE: PANICEAE) HUGO F. GUTIÉRREZ1 y OSVALDO MORRONE† Summary: Nomenclatural novelties in Cenchrus s.l. (Poaceae: Panicoideae: Paniceae). Recent phylogenetic studies with morphological and molecular data provided evidence on the monophyly of the genera Cenchrus, Pennisetum and Odontelytrum. Therefore, these studies propose the unification and transfer of species of Pennisetum and Odontelytrum to the genus Cenchrus, which has priority. Nomenclatural problems were detected when conducting a preliminary taxonomic revision of the genus Cenchrus s.l. from America (Gutiérrez, in preparation). To resolve these inconveniences, nine lectotypifications: Cenchrus bambusoides Caro & E.A. Sánchez, C. brevisetus E. Fourn., C. pennisetiformis Hochst. & Steud. var. intermedia Chiov., C. roseus E. Fourn., Gymnotrix mexicana E. Fourn., Hymenachne montana Griseb., Pennisetum amoenum Hochst. ex A. Rich., P. cenchroides Rich. var. hamphilahense Terracc., P. ciliare (L.) Link var. anachoreticum Chiov., P. petraeum Steud., P. pringlei Leeke, P. tristachyum (Kunth) Spreng. subsp. boliviense Chase and a new name for Gymnotrix crinita Kunth are here proposed. Key words: Cenchrus, Pennisetum, Poaceae, Paniceae, taxonomy. Resumen: Recientes estudios filogenéticos con datos morfológicos y moleculares aportaron evidencia sobre la monofilia de los géneros Cenchrus, Pennisetum y Odontelytrum y, por ello, se propuso su unificación y transferencia a Cenchrus, el cual tiene prioridad. Resultados preliminares de la revisión taxonómica del género Cenchrus s.l. para América (Gutiérrez, en preparación) permitieron detectar problemas nomenclaturales. Para resolver dichos inconvenientes, en el presente trabajo se presentan nueve lectotipificaciones: Cenchrus bambusoides Caro & E.A. -

Texas Coast Salt and Brackish Tidal Marsh
ECOLOGICAL MAPPING SYSTEMS OF TEXAS: TEXAS COAST SALT AND BRACKISH TIDAL MARSH TEXAS COAST SALT AND BRACKISH TIDAL MARSH Nature Serve ID: CES203.473 Geology: Recent alluvial and eolian deposits along the coast. Landform: Nearly level very gentle slopes, and flats influenced by tides. Soils: Coastal sands and various Salt Marsh Ecological Sites. Description: These marshes occupy relatively low-lying, coastal situations on level landforms influenced by tidal fluctuations. Some sites are only influenced by storm tides, or tides resulting from extreme wind events. The composition of these marshes is primarily influenced by the frequency and duration of tidal inundation. Salinity on some marshes, particularly in the south, is maintained by salt spray from prevailing southeasterly winds. Low marshes are regularly flooded and representative examples are dominated by Spartina alterniflora (smooth cordgrass), Juncus roemerianus (blackrush), or Avicennia germinans (black mangrove). Significant areas of Avicennia germinans (black mangrove) become more frequent towards the south, while extensive areas of Spartina alterniflora (smooth cordgrass) become rare south of Corpus Christi Bay. Areas of decreased frequency and/or duration of tidal inundation are often referred to as high, or irregularly flooded, marsh. These marshes may be dominated by species such as Spartina patens (marshhay cordgrass), Distichlis spicata (saltgrass), Schoenoplectus robustus (sturdy bulrush), Schoenoplectus americanus (three-square bulrush), Sporobolus virginicus (seashore dropseed), Monanthochloe littoralis (shoregrass), and Spartina spartinae (Gulf cordgrass). Shrubs, subshrubs, and forbs, such as Batis maritima (saltwort), Borrichia frutescens (sea ox-eye daisy), Sesuvium portulacastrum (shoreline seapurslane), Salicornia spp. (glassworts), Suaeda linearis (annual seepweed), Limonium spp. (sea-lavenders), and Lycium carolinianum (Carolina wolfberry) are commonly encountered in these marshes. -

A Guide on Common, Herbaceous, Hydrophytic Vegetation of Southern Texas
United States Department of Agriculture Natural Resources Conservation Service Technical Note No: TX-PM-20-02 July 2020 A Guide on Common, Herbaceous, Hydrophytic Vegetation of Southern Texas Plant Materials Technical Note Horsetail Background: Wetlands are those lands that have saturated soils, shallow standing water or flooding during at least a portion of the growing season. These sites have soils that are saturated for at least two consecutive weeks during the growing season and support a distinct vegetation type adapted for life in saturated soil conditions. Purpose: The purpose of this Technical Note is to provide information on the use of some common wetland plants of southern Texas. The list includes plants found along the Guadalupe River around Tivoli southward to the Rio Grande River floodplain. It is not intended to be a comprehensive treatment of the wetland flora of this region. Rather it is intended to introduce to the reader the many common wetland plant species that occur in south Texas. The guide is broken down into four categories: wildlife habitat, shoreline erosion control, water quality improvement and landscaping. Each species has a brief description of its identifying features, notes on its ecology or habitat, use and its National Wetlands Inventory (NWI) assessment. For more detailed information we suggest referring to our listed references. All pictures came from the USDA Plants Data Base or the E. “Kika” de la Garza Plant Materials Center. Plants for wildlife habitat: The plants listed in this section are primarily for waterbird and waterfowl habitat as well as for fish nursery and spawning areas. -

Chromosome Numbers in Compositae, XII: Heliantheae
SMITHSONIAN CONTRIBUTIONS TO BOTANY 0 NCTMBER 52 Chromosome Numbers in Compositae, XII: Heliantheae Harold Robinson, A. Michael Powell, Robert M. King, andJames F. Weedin SMITHSONIAN INSTITUTION PRESS City of Washington 1981 ABSTRACT Robinson, Harold, A. Michael Powell, Robert M. King, and James F. Weedin. Chromosome Numbers in Compositae, XII: Heliantheae. Smithsonian Contri- butions to Botany, number 52, 28 pages, 3 tables, 1981.-Chromosome reports are provided for 145 populations, including first reports for 33 species and three genera, Garcilassa, Riencourtia, and Helianthopsis. Chromosome numbers are arranged according to Robinson’s recently broadened concept of the Heliantheae, with citations for 212 of the ca. 265 genera and 32 of the 35 subtribes. Diverse elements, including the Ambrosieae, typical Heliantheae, most Helenieae, the Tegeteae, and genera such as Arnica from the Senecioneae, are seen to share a specialized cytological history involving polyploid ancestry. The authors disagree with one another regarding the point at which such polyploidy occurred and on whether subtribes lacking higher numbers, such as the Galinsoginae, share the polyploid ancestry. Numerous examples of aneuploid decrease, secondary polyploidy, and some secondary aneuploid decreases are cited. The Marshalliinae are considered remote from other subtribes and close to the Inuleae. Evidence from related tribes favors an ultimate base of X = 10 for the Heliantheae and at least the subfamily As teroideae. OFFICIALPUBLICATION DATE is handstamped in a limited number of initial copies and is recorded in the Institution’s annual report, Smithsonian Year. SERIESCOVER DESIGN: Leaf clearing from the katsura tree Cercidiphyllumjaponicum Siebold and Zuccarini. Library of Congress Cataloging in Publication Data Main entry under title: Chromosome numbers in Compositae, XII. -

Resolution of Deep Angiosperm Phylogeny Using Conserved Nuclear Genes and Estimates of Early Divergence Times
ARTICLE Received 24 Mar 2014 | Accepted 11 Aug 2014 | Published 24 Sep 2014 DOI: 10.1038/ncomms5956 OPEN Resolution of deep angiosperm phylogeny using conserved nuclear genes and estimates of early divergence times Liping Zeng1, Qiang Zhang2, Renran Sun1, Hongzhi Kong3, Ning Zhang1,4 & Hong Ma1,5 Angiosperms are the most successful plants and support human livelihood and ecosystems. Angiosperm phylogeny is the foundation of studies of gene function and phenotypic evolution, divergence time estimation and biogeography. The relationship of the five divergent groups of the Mesangiospermae (B99.95% of extant angiosperms) remains uncertain, with multiple hypotheses reported in the literature. Here transcriptome data sets are obtained from 26 species lacking sequenced genomes, representing each of the five groups: eudicots, monocots, magnoliids, Chloranthaceae and Ceratophyllaceae. Phylogenetic analyses using 59 carefully selected low-copy nuclear genes resulted in highly supported relationships: sisterhood of eudicots and a clade containing Chloranthaceae and Ceratophyllaceae, with magnoliids being the next sister group, followed by monocots. Our topology allows a re-examination of the evolutionary patterns of 110 morphological characters. The molecular clock estimates of Mesangiospermae diversification during the late to middle Jurassic correspond well to the origins of some insects, which may have been a factor facilitating early angiosperm radiation. 1 State Key Laboratory of Genetic Engineering and Collaborative Innovation Center for Genetics and Development, Ministry of Education Key Laboratoryof Biodiversity Sciences and Ecological Engineering, Institute of Plant Biology, Institute of Biodiversity Science, Center for Evolutionary Biology, School of Life Sciences, Fudan University, 220 Handan Road, Yangpu District, Shanghai 200433, China. 2 Guangxi Institute of Botany, Guangxi Zhuang Autonomous Region and the Chinese Academy of Sciences, Guilin 541006, China.