Helices and Spirals Helices in Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Engineering Curves – I
Engineering Curves – I 1. Classification 2. Conic sections - explanation 3. Common Definition 4. Ellipse – ( six methods of construction) 5. Parabola – ( Three methods of construction) 6. Hyperbola – ( Three methods of construction ) 7. Methods of drawing Tangents & Normals ( four cases) Engineering Curves – II 1. Classification 2. Definitions 3. Involutes - (five cases) 4. Cycloid 5. Trochoids – (Superior and Inferior) 6. Epic cycloid and Hypo - cycloid 7. Spiral (Two cases) 8. Helix – on cylinder & on cone 9. Methods of drawing Tangents and Normals (Three cases) ENGINEERING CURVES Part- I {Conic Sections} ELLIPSE PARABOLA HYPERBOLA 1.Concentric Circle Method 1.Rectangle Method 1.Rectangular Hyperbola (coordinates given) 2.Rectangle Method 2 Method of Tangents ( Triangle Method) 2 Rectangular Hyperbola 3.Oblong Method (P-V diagram - Equation given) 3.Basic Locus Method 4.Arcs of Circle Method (Directrix – focus) 3.Basic Locus Method (Directrix – focus) 5.Rhombus Metho 6.Basic Locus Method Methods of Drawing (Directrix – focus) Tangents & Normals To These Curves. CONIC SECTIONS ELLIPSE, PARABOLA AND HYPERBOLA ARE CALLED CONIC SECTIONS BECAUSE THESE CURVES APPEAR ON THE SURFACE OF A CONE WHEN IT IS CUT BY SOME TYPICAL CUTTING PLANES. OBSERVE ILLUSTRATIONS GIVEN BELOW.. Ellipse Section Plane Section Plane Hyperbola Through Generators Parallel to Axis. Section Plane Parallel to end generator. COMMON DEFINATION OF ELLIPSE, PARABOLA & HYPERBOLA: These are the loci of points moving in a plane such that the ratio of it’s distances from a fixed point And a fixed line always remains constant. The Ratio is called ECCENTRICITY. (E) A) For Ellipse E<1 B) For Parabola E=1 C) For Hyperbola E>1 Refer Problem nos. -

A Characterization of Helical Polynomial Curves of Any Degree
A CHARACTERIZATION OF HELICAL POLYNOMIAL CURVES OF ANY DEGREE J. MONTERDE Abstract. We give a full characterization of helical polynomial curves of any degree and a simple way to construct them. Existing results about Hermite interpolation are revisited. A simple method to select the best quintic interpolant among all possible solutions is suggested. 1. Introduction The notion of helical polynomial curves, i.e, polynomial curves which made a constant angle with a ¯xed line in space, have been studied by di®erent authors. Let us cite the papers [5, 6, 7] where the main results about the cubical and quintic cases are stablished. In [5] the authors gives a necessary condition a polynomial curve must satisfy in order to be a helix. The condition is expressed in terms of its hodograph, i.e., its derivative. If a polynomial curve, ®, is a helix then its hodograph, ®0, must be Pythagorean, i.e., jj®0jj2 is a perfect square of a polynomial. Moreover, this condition is su±cient in the cubical case: all PH cubical curves are helices. In the same paper it is also stated that not only ®0 must be Pythagorean, but also ®0^®00. Following the same ideas, in [1] it is proved that both conditions are su±cient in the quintic case. Unfortunately, this characterization is no longer true for higher degrees. It is possible to construct examples of polynomial curves of degree 7 verifying both conditions but being not a helix. The aim of this paper is just to show how a simple geometric trick can clarify the proofs of some previous results and to simplify the needed compu- tations to solve some related problems as for instance, the Hermite problem using helical polynomial curves. -

Linguistic Presentation of Objects
Zygmunt Ryznar dr emeritus Cracow Poland [email protected] Linguistic presentation of objects (types,structures,relations) Abstract Paper presents phrases for object specification in terms of structure, relations and dynamics. An applied notation provides a very concise (less words more contents) description of object. Keywords object relations, role, object type, object dynamics, geometric presentation Introduction This paper is based on OSL (Object Specification Language) [3] dedicated to present the various structures of objects, their relations and dynamics (events, actions and processes). Jay W.Forrester author of fundamental work "Industrial Dynamics”[4] considers the importance of relations: “the structure of interconnections and the interactions are often far more important than the parts of system”. Notation <!...> comment < > container of (phrase,name...) ≡> link to something external (outside of definition)) <def > </def> start-end of definition <spec> </spec> start-end of specification <beg> <end> start-end of section [..] or {..} 1 list of assigned keywords :[ or :{ structure ^<name> optional item (..) list of items xxxx(..) name of list = value assignment @ mark of special attribute,feature,property @dark unknown, to obtain, to discover :: belongs to : equivalent name (e.g.shortname) # number of |name| executive/operational object ppppXxxx name of item Xxxx with prefix ‘pppp ‘ XXXX basic object UUUU.xxxx xxxx object belonged to UUUU object class & / conjunctions ‘and’ ‘or’ 1 If using Latex editor we suggest [..] brackets -

Helical Curves and Double Pythagorean Hodographs
Helical polynomial curves and “double” Pythagorean-hodograph curves Rida T. Farouki Department of Mechanical & Aeronautical Engineering, University of California, Davis — synopsis — • introduction: properties of Pythagorean-hodograph curves • computing rotation-minimizing frames on spatial PH curves • helical polynomial space curves — are always PH curves • standard quaternion representation for spatial PH curves • “double” Pythagorean hodograph structure — requires both |r0(t)| and |r0(t) × r00(t)| to be polynomials in t • Hermite interpolation problem: selection of free parameters Pythagorean-hodograph (PH) curves r(ξ) = PH curve ⇐⇒ coordinate components of r0(ξ) comprise a “Pythagorean n-tuple of polynomials” in Rn PH curves incorporate special algebraic structures in their hodographs (complex number & quaternion models for planar & spatial PH curves) • rational offset curves rd(ξ) = r(ξ) + d n(ξ) Z ξ • polynomial arc-length function s(ξ) = |r0(ξ)| dξ 0 Z 1 • closed-form evaluation of energy integral E = κ2 ds 0 • real-time CNC interpolators, rotation-minimizing frames, etc. helical polynomial space curves several equivalent characterizations of helical curves • tangent t maintains constant inclination ψ with fixed vector a • a · t = cos ψ, where ψ = pitch angle and a = axis vector of helix • fixed curvature/torsion ratio, κ/τ = tan ψ (Theorem of Lancret) • curve has a circular tangent indicatrix on the unit sphere (small circle for space curve, great circle for planar curve) • (r(2) × r(3)) · r(4) ≡ 0 — where r(k) = kth arc–length derivative -

Study of Spiral Transition Curves As Related to the Visual Quality of Highway Alignment
A STUDY OF SPIRAL TRANSITION CURVES AS RELA'^^ED TO THE VISUAL QUALITY OF HIGHWAY ALIGNMENT JERRY SHELDON MURPHY B, S., Kansas State University, 1968 A MJvSTER'S THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Civil Engineering KANSAS STATE UNIVERSITY Manhattan, Kansas 1969 Approved by P^ajQT Professor TV- / / ^ / TABLE OF CONTENTS <2, 2^ INTRODUCTION 1 LITERATURE SEARCH 3 PURPOSE 5 SCOPE 6 • METHOD OF SOLUTION 7 RESULTS 18 RECOMMENDATIONS FOR FURTHER RESEARCH 27 CONCLUSION 33 REFERENCES 34 APPENDIX 36 LIST OF TABLES TABLE 1, Geonetry of Locations Studied 17 TABLE 2, Rates of Change of Slope Versus Curve Ratings 31 LIST OF FIGURES FIGURE 1. Definition of Sight Distance and Display Angle 8 FIGURE 2. Perspective Coordinate Transformation 9 FIGURE 3. Spiral Curve Calculation Equations 12 FIGURE 4. Flow Chart 14 FIGURE 5, Photograph and Perspective of Selected Location 15 FIGURE 6. Effect of Spiral Curves at Small Display Angles 19 A, No Spiral (Circular Curve) B, Completely Spiralized FIGURE 7. Effects of Spiral Curves (DA = .015 Radians, SD = 1000 Feet, D = l** and A = 10*) 20 Plate 1 A. No Spiral (Circular Curve) B, Spiral Length = 250 Feet FIGURE 8. Effects of Spiral Curves (DA = ,015 Radians, SD = 1000 Feet, D = 1° and A = 10°) 21 Plate 2 A. Spiral Length = 500 Feet B. Spiral Length = 1000 Feet (Conpletely Spiralized) FIGURE 9. Effects of Display Angle (D = 2°, A = 10°, Ig = 500 feet, = SD 500 feet) 23 Plate 1 A. Display Angle = .007 Radian B. Display Angle = .027 Radiaji FIGURE 10. -

Construction Surveying Curves
Construction Surveying Curves Three(3) Continuing Education Hours Course #LS1003 Approved Continuing Education for Licensed Professional Engineers EZ-pdh.com Ezekiel Enterprises, LLC 301 Mission Dr. Unit 571 New Smyrna Beach, FL 32170 800-433-1487 [email protected] Construction Surveying Curves Ezekiel Enterprises, LLC Course Description: The Construction Surveying Curves course satisfies three (3) hours of professional development. The course is designed as a distance learning course focused on the process required for a surveyor to establish curves. Objectives: The primary objective of this course is enable the student to understand practical methods to locate points along curves using variety of methods. Grading: Students must achieve a minimum score of 70% on the online quiz to pass this course. The quiz may be taken as many times as necessary to successful pass and complete the course. Ezekiel Enterprises, LLC Section I. Simple Horizontal Curves CURVE POINTS Simple The simple curve is an arc of a circle. It is the most By studying this course the surveyor learns to locate commonly used. The radius of the circle determines points using angles and distances. In construction the “sharpness” or “flatness” of the curve. The larger surveying, the surveyor must often establish the line of the radius, the “flatter” the curve. a curve for road layout or some other construction. The surveyor can establish curves of short radius, Compound usually less than one tape length, by holding one end Surveyors often have to use a compound curve because of the tape at the center of the circle and swinging the of the terrain. -

The Ordered Distribution of Natural Numbers on the Square Root Spiral
The Ordered Distribution of Natural Numbers on the Square Root Spiral - Harry K. Hahn - Ludwig-Erhard-Str. 10 D-76275 Et Germanytlingen, Germany ------------------------------ mathematical analysis by - Kay Schoenberger - Humboldt-University Berlin ----------------------------- 20. June 2007 Abstract : Natural numbers divisible by the same prime factor lie on defined spiral graphs which are running through the “Square Root Spiral“ ( also named as “Spiral of Theodorus” or “Wurzel Spirale“ or “Einstein Spiral” ). Prime Numbers also clearly accumulate on such spiral graphs. And the square numbers 4, 9, 16, 25, 36 … form a highly three-symmetrical system of three spiral graphs, which divide the square-root-spiral into three equal areas. A mathematical analysis shows that these spiral graphs are defined by quadratic polynomials. The Square Root Spiral is a geometrical structure which is based on the three basic constants: 1, sqrt2 and π (pi) , and the continuous application of the Pythagorean Theorem of the right angled triangle. Fibonacci number sequences also play a part in the structure of the Square Root Spiral. Fibonacci Numbers divide the Square Root Spiral into areas and angle sectors with constant proportions. These proportions are linked to the “golden mean” ( golden section ), which behaves as a self-avoiding-walk- constant in the lattice-like structure of the square root spiral. Contents of the general section Page 1 Introduction to the Square Root Spiral 2 2 Mathematical description of the Square Root Spiral 4 3 The distribution -

Chapter 2. Parameterized Curves in R3
Chapter 2. Parameterized Curves in R3 Def. A smooth curve in R3 is a smooth map σ :(a, b) → R3. For each t ∈ (a, b), σ(t) ∈ R3. As t increases from a to b, σ(t) traces out a curve in R3. In terms of components, σ(t) = (x(t), y(t), z(t)) , (1) or x = x(t) σ : y = y(t) a < t < b , z = z(t) dσ velocity at time t: (t) = σ0(t) = (x0(t), y0(t), z0(t)) . dt dσ speed at time t: (t) = |σ0(t)| dt Ex. σ : R → R3, σ(t) = (r cos t, r sin t, 0) - the standard parameterization of the unit circle, x = r cos t σ : y = r sin t z = 0 σ0(t) = (−r sin t, r cos t, 0) |σ0(t)| = r (constant speed) 1 Ex. σ : R → R3, σ(t) = (r cos t, r sin t, ht), r, h > 0 constants (helix). σ0(t) = (−r sin t, r cos t, h) √ |σ0(t)| = r2 + h2 (constant) Def A regular curve in R3 is a smooth curve σ :(a, b) → R3 such that σ0(t) 6= 0 for all t ∈ (a, b). That is, a regular curve is a smooth curve with everywhere nonzero velocity. Ex. Examples above are regular. Ex. σ : R → R3, σ(t) = (t3, t2, 0). σ is smooth, but not regular: σ0(t) = (3t2, 2t, 0) , σ0(0) = (0, 0, 0) Graph: x = t3 y = t2 = (x1/3)2 σ : y = t2 ⇒ y = x2/3 z = 0 There is a cusp, not because the curve isn’t smooth, but because the velocity = 0 at the origin. -

Archimedean Spirals ∗
Archimedean Spirals ∗ An Archimedean Spiral is a curve defined by a polar equation of the form r = θa, with special names being given for certain values of a. For example if a = 1, so r = θ, then it is called Archimedes’ Spiral. Archimede’s Spiral For a = −1, so r = 1/θ, we get the reciprocal (or hyperbolic) spiral. Reciprocal Spiral ∗This file is from the 3D-XploreMath project. You can find it on the web by searching the name. 1 √ The case a = 1/2, so r = θ, is called the Fermat (or hyperbolic) spiral. Fermat’s Spiral √ While a = −1/2, or r = 1/ θ, it is called the Lituus. Lituus In 3D-XplorMath, you can change the parameter a by going to the menu Settings → Set Parameters, and change the value of aa. You can see an animation of Archimedean spirals where the exponent a varies gradually, from the menu Animate → Morph. 2 The reason that the parabolic spiral and the hyperbolic spiral are so named is that their equations in polar coordinates, rθ = 1 and r2 = θ, respectively resembles the equations for a hyperbola (xy = 1) and parabola (x2 = y) in rectangular coordinates. The hyperbolic spiral is also called reciprocal spiral because it is the inverse curve of Archimedes’ spiral, with inversion center at the origin. The inversion curve of any Archimedean spirals with respect to a circle as center is another Archimedean spiral, scaled by the square of the radius of the circle. This is easily seen as follows. If a point P in the plane has polar coordinates (r, θ), then under inversion in the circle of radius b centered at the origin, it gets mapped to the point P 0 with polar coordinates (b2/r, θ), so that points having polar coordinates (ta, θ) are mapped to points having polar coordinates (b2t−a, θ). -
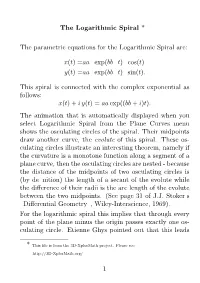
The Logarithmic Spiral * the Parametric Equations for The
The Logarithmic Spiral * The parametric equations for the Logarithmic Spiral are: x(t) =aa exp(bb t) cos(t) · · · y(t) =aa exp(bb t) sin(t). · · · This spiral is connected with the complex exponential as follows: x(t) + i y(t) = aa exp((bb + i)t). The animation that is automatically displayed when you select Logarithmic Spiral from the Plane Curves menu shows the osculating circles of the spiral. Their midpoints draw another curve, the evolute of this spiral. These os- culating circles illustrate an interesting theorem, namely if the curvature is a monotone function along a segment of a plane curve, then the osculating circles are nested - because the distance of the midpoints of two osculating circles is (by definition) the length of a secant of the evolute while the difference of their radii is the arc length of the evolute between the two midpoints. (See page 31 of J.J. Stoker’s “Differential Geometry”, Wiley-Interscience, 1969). For the logarithmic spiral this implies that through every point of the plane minus the origin passes exactly one os- culating circle. Etienne´ Ghys pointed out that this leads * This file is from the 3D-XplorMath project. Please see: http://3D-XplorMath.org/ 1 to a surprise: The unit tangent vectors of the osculating circles define a vector field X on R2 0 – but this vec- tor field has more integral curves, i.e.\ {solution} curves of the ODE c0(t) = X(c(t)), than just the osculating circles, namely also the logarithmic spiral. How is this compatible with the uniqueness results of ODE solutions? Read words backwards for explanation: eht dleifrotcev si ton ztihcspiL gnola eht evruc. -

On Helices and Bertrand Curves in Euclidean 3-Space
ON HELİCES AND BERTRAND CURVES IN EUCLİDEAN 3-SPACE Murat Babaarslan Department of Mathematics, Faculty of Science and Arts Bozok University, Yozgat, Turkey [email protected] Yusuf Yaylı Department of Mathematics, Faculty of Science Ankara University, Tandoğan, Ankara, Turkey [email protected] Abstract- In this article, we investigate Bertrand curves corresponding to spherical images of the tangent indicatrix, binormal indicatrix, principal normal indicatrix and Darboux indicatrix of a space curve in Euclidean 3-space. As a result, in case of a space curve is general helix, we show that curve corresponding to spherical images of its tangent indicatrix and binormal indicatrix are circular helices and Bertrand curves. Similarly, in case of a space curve is slant helix, we demonstrate that the curve corresponding to spherical image of its principal normal indicatrix is circular helix and Bertrand curve. Key Words- Helix, Bertrand curve, Spherical Images 1. INTRODUCTION In the differential geometry of a regular curve in Euclidean 3-space E3 , it is well known that, one of the important problems is characterization of a regular curve. The curvature and the torsion of a regular curve play an important role to determine the shape and size of the curve 1,2. Natural scientists have long held a fascination, sometimes bordering on mystical obsession for helical structures in nature. Helices arise in nanosprings, carbon nonotubes, helices, DNA double and collagen triple helix, the double helix shape is commonly associated with DNA, since the double helix is structure of DNA 3 . This fact was published for the first time by Watson and Crick in 1953 4. -

Effect of Superhelical Structure on the Secondary Structure of DNA Rings
VOL. 5, PP. 691-696 (1967) Effect of Superhelical Structure on the Secondary Structure of DNA Rings DANIEL GLAUBIGER and JOHN E. HEARST, Departnient of Chemistry, University of California, Berkeley, California Synopsis A quantity, called the linking number, is defined, which specifies the total number of t,wists in a circular helix. The linking number is invariant under continuous deforma- tions of the ring and therefore enables one to calculate the influence of superhelical structures on the secondary helix of a circular molecule. The linking number can be determined by projecting the helix into a plane and counting strand crosses in the projection as described. For example, it has been shown that for each 180" twist in a left-handed superhelix, a right-handed 360" twist is removed from the secondary helix, thus allowing local unwinding. Introduction It is of interest to relate superhelical structure of helical molecules to their secondary structure.' By defining a quantity characteristic of two- stranded circular helical niolecules, related to the helical structure, and in- variant under continuous deformations of the molecule, we are able to evaluate the changes in helical structure brought about by the imposition of superhelical configurations on such molecules. The quantity of interest, called the linking number, is related to the number of turns/cycle for a circular helical molecule.* It will be shown that superhelical structures which are made from helical polymers con- tribute to this number and alter the contribution made by the secondary structure of the molecule to this number. This is made manifest as a change in the number of turns/cycle or as a change in the number of turns/ unit length.