The Pineal Gland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of the Hypothalamic Dorsomedial Nucleus in the Central Regulation of Food Intake
The role of the hypothalamic dorsomedial nucleus in the central regulation of food intake Ph.D. thesis Éva Dobolyiné Renner Semmelweis University Szentágothai János Neuroscience Doctoral School Ph.D. Supervisor: Prof. Miklós Palkovits, member of the HAS Opponents: Prof. Dr. Halasy Katalin, Ph.D., D.Sci. Dr. Oláh Márk, M.D., Ph.D. Examination board: Prof. Dr. Vígh Béla, M.D., Ph.D., D.Sci Dr. Kovács Krisztina Judit, Ph.D., D.Sci. Dr. Lovas Gábor, M.D., Ph.D. Budapest 2013 1. Introduction The central role of the hypothalamus in the regulation of food intake and energy expenditure has long been established. The hypothalamus receives hormonal input such as insulin, leptin, and ghrelin from the periphery. The gate for the most important adiposity signals is the arcuate nucleus, which contains neurons expressing orexigenic and anorexigenic peptides, respectively. These neurons convey peripheral input to the paraventricular and ventromedial nuclei, and the lateral hypothalamic area, which all play critical roles in body weight regulations. The hypothalamic dorsomedial nucleus (DMH) has also been implicated in the regulation of body weight homeostasis along with other hypothalamic nuclei including the arcuate, ventromedial, and paraventricular nuclei as well as the lateral hypothalamus. Lesions of the DMH affected ingestive behavior. Electrophysiological data suggested that neurons in this nucleus integrate hormonal input and ascending brainstem information and, in turn, modulate food intake and energy balance. In response to refeeding of fasted rats, Fos-activated neurons were reported in the DMH. Major projections relay vagus-mediated signals from the gastrointestinal tract, and humoral signals to the hypothalamus from the nucleus of the solitary tract (NTS), a viscerosensory cell group in the dorsomedial medulla. -

Cortex and Thalamus Lecture.Pptx
Cerebral Cortex and Thalamus Hyperbrain Ch 2 Monica Vetter, PhD January 24, 2013 Learning Objectives: • Anatomy of the lobes of the cortex • Relationship of thalamus to cortex • Layers and connectivity of the cortex • Vascular supply to cortex • Understand the location and function of hypothalamus and pituitary • Anatomy of the basal ganglia • Primary functions of the different lobes/ cortical regions – neurological findings 1 Types of Cortex • Sensory (Primary) • Motor (Primary) • Unimodal association • Multimodal association - necessary for language, reason, plan, imagine, create Note: • Gyri • Sulci • Fissures • Lobes 2 The Thalamus is highly interconnected with the cerebral cortex, and handles most information traveling to or from the cortex. “Specific thalamic Ignore nuclei” – have well- names of defined sensory or thalamic nuclei for motor functions now - A few Other nuclei have will more distributed reappear later function 3 Thalamus Midbrain Pons Limbic lobe = cingulate gyrus Structure of Neocortex (6 layers) white matter gray matter Pyramidal cells 4 Connectivity of neurons in different cortical layers Afferents = inputs Efferents = outputs (reciprocal) brainstem etc Eg. Motor – Eg. Sensory – more efferent more afferent output input Cortico- cortical From Thalamus To spinal cord, brainstem etc. To Thalamus Afferent and efferent connections to different ….Depending on whether they have more layers of cortex afferent or efferent connections 5 Different areas of cortex were defined by differences in layer thickness, and size and -

Critical Role of Dorsomedial Hypothalamic Nucleus in a Wide Range of Behavioral Circadian Rhythms
The Journal of Neuroscience, November 19, 2003 • 23(33):10691–10702 • 10691 Behavioral/Systems/Cognitive Critical Role of Dorsomedial Hypothalamic Nucleus in a Wide Range of Behavioral Circadian Rhythms Thomas C. Chou,1,2 Thomas E. Scammell,2 Joshua J. Gooley,1,2 Stephanie E. Gaus,1,2 Clifford B. Saper,2 and Jun Lu2 1Department of Neurobiology and Program in Neuroscience, Harvard Medical School, Boston, Massachusetts 02115, and 2Department of Neurology, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, Massachusetts 02215 The suprachiasmatic nucleus (SCN) contains the brain’s circadian pacemaker, but mechanisms by which it controls circadian rhythms of sleep and related behaviors are poorly understood. Previous anatomic evidence has implicated the dorsomedial hypothalamic nucleus (DMH) in circadian control of sleep, but this hypothesis remains untested. We now show that excitotoxic lesions of the DMH reduce circadian rhythms of wakefulness, feeding, locomotor activity, and serum corticosteroid levels by 78–89% while also reducing their overall daily levels. We also show that the DMH receives both direct and indirect SCN inputs and sends a mainly GABAergic projection to the sleep-promoting ventrolateral preoptic nucleus, and a mainly glutamate–thyrotropin-releasing hormone projection to the wake- promoting lateral hypothalamic area, including orexin (hypocretin) neurons. Through these pathways, the DMH may influence a wide range of behavioral circadian rhythms. Key words: suprachiasmatic nucleus; sleep; feeding; corticosteroid; locomotor activity; melatonin Introduction sponses, in feeding, and in the circadian release of corticosteroids In many animals, the suprachiasmatic nucleus (SCN) strongly (for review, see Bellinger et al., 1976; Kalsbeek et al., 1996; Ber- influences circadian rhythms of sleep–wake behaviors, but the nardis and Bellinger, 1998; DiMicco et al., 2002), although many pathways mediating these influences are poorly understood. -

Aversive Stimuli Drive Hypothalamus-To-Habenula
Aversive stimuli drive hypothalamus-to-habenula excitation to promote escape behavior Salvatore Lecca, Frank Meye, Massimo Trusel, Anna Tchenio, Julia Harris, Martin Karl Schwarz, Denis Burdakov, F Georges, Manuel Mameli To cite this version: Salvatore Lecca, Frank Meye, Massimo Trusel, Anna Tchenio, Julia Harris, et al.. Aversive stimuli drive hypothalamus-to-habenula excitation to promote escape behavior. eLife, eLife Sciences Publication, 2017, 6, pp.e30697. 10.7554/eLife.30697. hal-03129952 HAL Id: hal-03129952 https://hal.archives-ouvertes.fr/hal-03129952 Submitted on 3 Feb 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. SHORT REPORT Aversive stimuli drive hypothalamus-to- habenula excitation to promote escape behavior Salvatore Lecca1,2, Frank Julius Meye1,3, Massimo Trusel1,2, Anna Tchenio1,2, Julia Harris4, Martin Karl Schwarz5, Denis Burdakov4, Francois Georges6,7, Manuel Mameli1,2* 1Institut du Fer a` Moulin, Inserm UMR-S 839, Paris, France; 2Department of Fundamental Neuroscience, The University of Lausanne, Lausanne, Switzerland; -

The Potential Therapeutic Effect of Melatonin in Gastro-Esophageal Reflux Disease Tharwat S Kandil1*, Amany a Mousa2, Ahmed a El-Gendy3, Amr M Abbas3
Kandil et al. BMC Gastroenterology 2010, 10:7 http://www.biomedcentral.com/1471-230X/10/7 RESEARCH ARTICLE Open Access The potential therapeutic effect of melatonin in gastro-esophageal reflux disease Tharwat S Kandil1*, Amany A Mousa2, Ahmed A El-Gendy3, Amr M Abbas3 Abstract Background: Gastro-Esophageal Reflux Disease (GERD) defined as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications. Many drugs are used for the treatment of GERD such as omeprazole (a proton pump inhibitor) which is a widely used antiulcer drug demonstrated to protect against esophageal mucosal injury. Melatonin has been found to protect the gastrointestinal mucosa from oxidative damage caused by reactive oxygen species in different experimental ulcer models. The aim of this study is to evaluate the role of exogenous melatonin in the treatment of reflux disease in humans either alone or in combination with omeprazole therapy. Methods: 36 persons were divided into 4 groups (control subjects, patients with reflux disease treated with melatonin alone, omeprazole alone and a combination of melatonin and omeprazole for 4 and 8 weeks) Each group consisted of 9 persons. Persons were subjected to thorough history taking, clinical examination, and investigations including laboratory, endoscopic, record of esophageal motility, pH-metry, basal acid output and serum gastrin. Results: Melatonin has a role in the improvement of Gastro-esophageal reflux disease when used alone or in combination with omeprazole. Meanwhile, omeprazole alone is better used in the treatment of GERD than melatonin alone. Conclusion: The present study showed that oral melatonin is a promising therapeutic agent for the treatment of GERD. -

07. Endocrine, Reproductive and Urogenital Pharmacology 07.001
07. Endocrine, Reproductive and Urogenital Pharmacology 07.001 Mirabegron relaxes urethral smooth muscle by a dual mechanism involving β3-Adrenoceptor activation and α1-adrenoceptor blockade. Alexandre EC1, Kiguti LR2, Calmasini FB1, Ferreira R3, Silva FH1, Silva KP2, Ribeiro CA2, Mónica FZ1, Pupo AS2, Antunes E1 1FCM-Unicamp – Farmacologia, 2IBB-Unesp, 3FCM- Unicamp – Hematologia e Hemoterapia Introduction: Overactive bladder syndrome (OAB) is a subset of storage LUTS (lower urinary tract symptoms) highly prevalent in diabetes, obesity and hypertension. Benign prostatic hyperplasia (BPH) in aging men is another pathological condition highly associated with OAB secondary to bladder outlet obstruction (BOO). The β3- adrenoceptor apparently is the major receptor to induce bladder relaxations. Mirabegron is the first β3-adrenoceptor (β3-AR) agonist approved for OAB treatment (Chapple et al., 2014). Urethral smooth muscle plays a critical role to urinary continence, but no studies have examined the mirabegron-induced urethral relaxations. Aims: This study was designed to investigate the mirabegron-induced mouse urethral relaxations. In preliminary assays, mirabegron showed an unexpected action by competitively antagonizing the urethral contractions induced by the α1-AR agonist phenylephrine. Therefore, this study also aimed to characterize the α1-AR blockade by mirabegron, focusing on the α1-AR subtypes in rat vas deferens and prostate (α1A- AR), spleen (α1B-AR) and aorta (α1D-AR) preparations. Methods: Functional assays were carried out in mouse urethra rings, and rat vas deferens, prostate, aorta and spleen. β3-AR expression (mRNA and immunohistochemistry) and cyclic AMP levels were determined in mouse urethra. Competition assays for the specific binding of [3H]Prazosin to membrane preparations of HEK 293 cells expressing each of the human α1-ARs subtypes were performed. -

1-Anatomy of the Pituitary Gland
Color Code Important Anatomy of Pituitary Gland Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, students should be able to: ✓ Describe the position of the pituitary gland. ✓ List the structures related to the pituitary gland. ✓ Differentiate between the lobes of the gland. ✓ Describe the blood supply of pituitary gland & the hypophyseal portal system. الغدة النخامية Pituitary Gland (also called Hypophysis Cerebri) o It is referred to as the master of endocrine glands. o It is a small oval structure 1 cm in diameter. o It doubles its size during pregnancy. lactation ,(الحمل) pregnancy ,(الحيض) A women experiences changes in her hormone levels during menstruation But only the pituitary gland will only increase in size during pregnancy .(سن اليأس) and menopause ,(الرضاعة) X-RAY SKULL: LATERAL VIEW SAGITTAL SECTION OF HEAD & NECK Extra Pituitary Gland Position o It lies in the middle cranial fossa. o It is well protected in sella turcica* (hypophyseal fossa) of body of sphenoid o It lies between optic chiasma (anteriorly) & mamillary bodies** (posteriorly). Clinical point: *سرج الحصان Anterior to the pituitary gland is the optic chiasm, so if there was a tumor in the pituitary gland or it was ** Part of hypothalamus enlarged this could press on the chiasm and disrupt the patients vision (loss of temporal field). Extra Pictures The purple part is the sphenoid bone Hypophyseal fossa Pituitary Gland The relations are important Important Relations • SUPERIOR: Diaphragma sellae: A fold of dura mater covers the pituitary gland & has an opening for passage of infundibulum (pituitary stalk) connecting the gland to hypothalamus. -
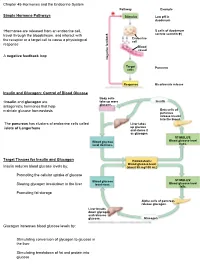
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -

Glossary of Pediatric Endocrine Terms
Glossary of Pediatric Endocrine Terms Adrenal Glands: Triangle-shaped glands located on top of each kidney that are responsible for the regulation of stress response by producing hormones such as cortisol and adrenaline. Adrenal Crisis: A life-threatening condition that results when there is not enough cortisol (stress hormone) in the body. This may present with nausea, vomiting, abdominal pain, severely low blood pressure, and loss of consciousness. Adrenocorticotropin (ACTH): A hormone produced by the anterior pituitary gland that triggers the adrenal gland to produce cortisol, the stress hormone. Anterior Pituitary: The front of the pituitary gland that secretes growth hormone, gonadotropins, thyroid stimulating hormone, prolactin, adrenocorticotropin hormone. Antidiuretic Hormone: A hormone secreted by the posterior pituitary that controls how much water is excreted by the kidneys (water balance). Bone Age Study: An X-ray of the left hand and wrist to assess a child’s skeletal age. It is often used to evaluate disorders of puberty and growth. Congenital Adrenal Hyperplasia: A group of disorders which impair the adrenal steroid synthesis pathway. This causes the body makes too many androgens. Sometimes the body does not make enough cortisol and aldosterone. Constitutional Delay of Growth and Puberty: A normal variant of growth in which children grow at a slower rate and begin puberty later than their peers. They may appear short until they have catch up growth. They usually end up at a normal adult height. A diagnosis of constitutional delay of growth and puberty is often referred to as being a “late bloomer.” Cortisol: The “stress hormone”, which is produced by the adrenal glands. -

2018 Camp Lesson Book
Arkansas 4-H Veterinary Science Urinalysis 1 Why Urine? Urine is the end product of a filtering process that removes waste from the body The color of urine can give you information about hydration level as well as possible underlying disease A urinalysis should be performed at least yearly for healthy pets, and more often for older animals and those with existing or chronic health issues Important elements of a urinalysis include a visual inspection of the urine sample, a dipstick test, and microscopic evaluation of urine sediment 2 The Urinary System The urinary tract consists of the kidneys, the ureters, the bladder, the urethra, and finally, the urethral opening at either the end of the penis or just within the vagina Kidneys filter out waste products from the blood Ureters connect the kidneys to the bladder The urethra is a tube that is controlled by a sphincter muscle that empties the bladder to the outside world 3 The Bladder Detrusor muscle Ureter Bladder Ureteral Opening Bladder Neck Sphincter Muscles Trigone Urethra 4 Urinary Tract Problems Inflammation of bladder caused by stress Bacterial or fungal bladder infections Inflammation of bladder from urinary crystals Inflammation of bladder from bladder stones Inflammation of the urethra Damage to ureters by trauma, passing kidney stones, surgical accident or cancer Damage to kidneys by dehydration, infection, toxins or cancer 5 Feline Idiopathic Cystitis Inflammation of the bladder with an unknown cause Can quickly lead to kidney and heart problems Can lead to -

The Third Eye and Pineal Gland Connection
D.U.Quark Volume 5 Issue 1 Fall 2020 Article 2 12-27-2020 Rolling My Third Eye: The Third Eye and Pineal Gland Connection Shannon B. Jackson Follow this and additional works at: https://dsc.duq.edu/duquark Recommended Citation Jackson, S. B. (2020). Rolling My Third Eye: The Third Eye and Pineal Gland Connection. D.U.Quark, 5 (1). Retrieved from https://dsc.duq.edu/duquark/vol5/iss1/2 This Staff Piece is brought to you for free and open access by Duquesne Scholarship Collection. It has been accepted for inclusion in D.U.Quark by an authorized editor of Duquesne Scholarship Collection. Rolling My Third Eye: The Third Eye and Pineal Gland Connection By Shannon Bow Jackson D.U.Quark 2020. Volume 5 (Issue 1) pgs. 6-13 Published December 27, 2020 Staff Article Chances are the optometrist only checks that two of your eyes are functioning. But what about your third eye; who checks on that? A neurologist? Spiritual Healer? Yoga Instructor? Yourself? The answer might vary, given that this third eye is believed to reside within the pineal gland inside of the brain. The name “third eye” comes from the pineal gland’s primary function of ‘letting in light and darkness’, just as our two eyes do. This gland is the melatonin-secreting neuroendocrine organ containing light-sensitive cells that control the circadian rhythm (1). The diagram shows that nerve cells in the retinas of our eyes allow for light to be sensed. When there is light, the nerve cells in the retina then signal to the suprachiasmatic nucleus (SCN) in the hypothalamus. -

Tonic Activity in Lateral Habenula Neurons Promotes Disengagement from Reward-Seeking Behavior
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.15.426914; this version posted January 16, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Tonic activity in lateral habenula neurons promotes disengagement from reward-seeking behavior 5 Brianna J. Sleezer1,3, Ryan J. Post1,2,3, David A. Bulkin1,2,3, R. Becket Ebitz4, Vladlena Lee1, Kasey Han1, Melissa R. Warden1,2,5,* 1Department of Neurobiology and Behavior, Cornell University, Ithaca, NY 14853, USA 2Cornell Neurotech, Cornell University, Ithaca, NY 14853 USA 10 3These authors contributed equally 4Department oF Neuroscience, Université de Montréal, Montréal, QC H3T 1J4, Canada 5Lead Contact *Correspondence: [email protected] 15 Sleezer*, Post*, Bulkin* et al. 1 of 38 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.15.426914; this version posted January 16, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. SUMMARY Survival requires both the ability to persistently pursue goals and the ability to determine when it is time to stop, an adaptive balance of perseverance and disengagement. Neural activity in the 5 lateral habenula (LHb) has been linked to aversion and negative valence, but its role in regulating the balance between reward-seeking and disengaged behavioral states remains unclear.