Changes in Macromoth Community Structure Following Deforestation in Western Washington State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
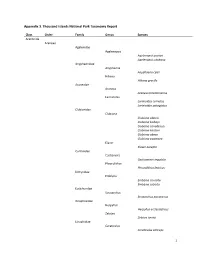
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Survey of the Lepidoptera Fauna in Birch Mountains Wildland Provincial Park
Survey of the Lepidoptera Fauna in Birch Mountains Wildland Provincial Park Platarctia parthenos Photo: D. Vujnovic Prepared for: Alberta Natural Heritage Information Centre, Parks and Protected Areas Division, Alberta Community Development Prepared by: Doug Macaulay and Greg Pohl Alberta Lepidopterists' Guild May 10, 2005 Figure 1. Doug Macaulay and Gerald Hilchie walking on a cutline near site 26. (Photo by Stacy Macaulay) Figure 2. Stacey Macaulay crossing a beaver dam at site 33. (Photo by Doug Macaulay) I TABLE OF CONTENTS INTRODUCTION................................................................................................................... 1 METHODS .............................................................................................................................. 1 RESULTS ................................................................................................................................ 3 DISCUSSION .......................................................................................................................... 4 I. Factors affecting the Survey...........................................................................................4 II. Taxa of particular interest.............................................................................................5 A. Butterflies:...................................................................................................................... 5 B. Macro-moths .................................................................................................................. -

Big Creek Lepidoptera Checklist
Big Creek Lepidoptera Checklist Prepared by J.A. Powell, Essig Museum of Entomology, UC Berkeley. For a description of the Big Creek Lepidoptera Survey, see Powell, J.A. Big Creek Reserve Lepidoptera Survey: Recovery of Populations after the 1985 Rat Creek Fire. In Views of a Coastal Wilderness: 20 Years of Research at Big Creek Reserve. (copies available at the reserve). family genus species subspecies author Acrolepiidae Acrolepiopsis californica Gaedicke Adelidae Adela flammeusella Chambers Adelidae Adela punctiferella Walsingham Adelidae Adela septentrionella Walsingham Adelidae Adela trigrapha Zeller Alucitidae Alucita hexadactyla Linnaeus Arctiidae Apantesis ornata (Packard) Arctiidae Apantesis proxima (Guerin-Meneville) Arctiidae Arachnis picta Packard Arctiidae Cisthene deserta (Felder) Arctiidae Cisthene faustinula (Boisduval) Arctiidae Cisthene liberomacula (Dyar) Arctiidae Gnophaela latipennis (Boisduval) Arctiidae Hemihyalea edwardsii (Packard) Arctiidae Lophocampa maculata Harris Arctiidae Lycomorpha grotei (Packard) Arctiidae Spilosoma vagans (Boisduval) Arctiidae Spilosoma vestalis Packard Argyresthiidae Argyresthia cupressella Walsingham Argyresthiidae Argyresthia franciscella Busck Argyresthiidae Argyresthia sp. (gray) Blastobasidae ?genus Blastobasidae Blastobasis ?glandulella (Riley) Blastobasidae Holcocera (sp.1) Blastobasidae Holcocera (sp.2) Blastobasidae Holcocera (sp.3) Blastobasidae Holcocera (sp.4) Blastobasidae Holcocera (sp.5) Blastobasidae Holcocera (sp.6) Blastobasidae Holcocera gigantella (Chambers) Blastobasidae -

Macrolepidoptera Inventory of the Chilcotin District
Macrolepidoptera Inventory of the Chilcotin District Aud I. Fischer – Biologist Jon H. Shepard - Research Scientist and Crispin S. Guppy – Research Scientist January 31, 2000 2 Abstract This study was undertaken to learn more of the distribution, status and habitat requirements of B.C. macrolepidoptera (butterflies and the larger moths), the group of insects given the highest priority by the BC Environment Conservation Center. The study was conducted in the Chilcotin District near Williams Lake and Riske Creek in central B.C. The study area contains a wide variety of habitats, including rare habitat types that elsewhere occur only in the Lillooet-Lytton area of the Fraser Canyon and, in some cases, the Southern Interior. Specimens were collected with light traps and by aerial net. A total of 538 species of macrolepidoptera were identified during the two years of the project, which is 96% of the estimated total number of species in the study area. There were 29,689 specimens collected, and 9,988 records of the number of specimens of each species captured on each date at each sample site. A list of the species recorded from the Chilcotin is provided, with a summary of provincial and global distributions. The habitats, at site series level as TEM mapped, are provided for each sample. A subset of the data was provided to the Ministry of Forests (Research Section, Williams Lake) for use in a Flamulated Owl study. A voucher collection of 2,526 moth and butterfly specimens was deposited in the Royal BC Museum. There were 25 species that are rare in BC, with most known only from the Riske Creek area. -

Survey of Lepidoptera of the Wainwright Dunes Ecological Reserve
SURVEY OF LEPIDOPTERA OF THE WAINWRIGHT DUNES ECOLOGICAL RESERVE Alberta Species at Risk Report No. 159 SURVEY OF LEPIDOPTERA OF THE WAINWRIGHT DUNES ECOLOGICAL RESERVE Doug Macaulay Alberta Species at Risk Report No.159 Project Partners: i ISBN 978-1-4601-3449-8 ISSN 1496-7146 Photo: Doug Macaulay of Pale Yellow Dune Moth ( Copablepharon grandis ) For copies of this report, visit our website at: http://www.aep.gov.ab.ca/fw/speciesatrisk/index.html This publication may be cited as: Macaulay, A. D. 2016. Survey of Lepidoptera of the Wainwright Dunes Ecological Reserve. Alberta Species at Risk Report No.159. Alberta Environment and Parks, Edmonton, AB. 31 pp. ii DISCLAIMER The views and opinions expressed are those of the authors and do not necessarily represent the policies of the Department or the Alberta Government. iii Table of Contents ACKNOWLEDGEMENTS ............................................................................................... vi EXECUTIVE SUMMARY ............................................................................................... vi 1.0 Introduction ................................................................................................................... 1 2.0 STUDY AREA ............................................................................................................. 2 3.0 METHODS ................................................................................................................... 6 4.0 RESULTS .................................................................................................................... -

TWO NEW SPECIES of MOTHS (NOCTUIDAE: ACRONICTINAE, CUCULLIINAE) from MIDLAND UNITED STATES Since Their Origins, the Ohio Lepidop
Journal of the Lepidopterists' Society 46(3), 1992, 220-232 TWO NEW SPECIES OF MOTHS (NOCTUIDAE: ACRONICTINAE, CUCULLIINAE) FROM MIDLAND UNITED STATES CHARLES y, COVELL JR. Department of Biology, University of Louisville, Louisville, Kentucky 40292 AND ERIC H. METZLER Ohio Department of Natural Resources, 1952 Belcher Drive, Columbus, Ohio 43224 ABSTRACT. Two new species of noctuid moths are described and illustrated. Ac ronicta heitzmani, new species, in the subfamily Acronictinae, is known from Missouri, Arkansas, Illinois and Ohio. Lithophane joannis, new species, in the subfamily Cucul Iiinae, is known from Ohio, Kentucky, and Michigan. Both species are compared with morphologically similar congeners. Additional key words: Acronicta heitzmani, Lithophane joannis, faunal survey. Since their origins, the Ohio Lepidopterists and the Society of Ken tucky Lepidopterists ha ve promoted regional surveys of the Lepidoptera fauna of midland United States. These efforts have resulted in numerous new records and range extensions and in the discovery of several new taxa, The purpose of this paper is to describe and illustrate two recently discovered species of the family Noctuidae. Both apparently are re stricted to midland United States, Acronicta heitzmani, new species, is known from Missouri, Arkansas, Illinois and Ohio. Lithophane joan nis, new species, is known from Ohio, Kentucky, and Michigan. Both species are morphologically distinct from, and sympatric with, con geners, In 1964, J. R, Heitzman collected a series of an unusual Acronicta species in Missouri. The specimens superficially resembled A. fragilis (Guenee) which was not recorded from Missouri. In 1967, the first author collected a specimen of the same species in Kentucky; the second author took the first Ohio specimen in 1975, The specimens were de termined as a possibly undescribed species near A. -

Zoogeography of the Holarctic Species of the Noctuidae (Lepidoptera): Importance of the Bering Ian Refuge
© Entomologica Fennica. 8.XI.l991 Zoogeography of the Holarctic species of the Noctuidae (Lepidoptera): importance of the Bering ian refuge Kauri Mikkola, J, D. Lafontaine & V. S. Kononenko Mikkola, K., Lafontaine, J.D. & Kononenko, V. S. 1991 : Zoogeography of the Holarctic species of the Noctuidae (Lepidoptera): importance of the Beringian refuge. - En to mol. Fennica 2: 157- 173. As a result of published and unpublished revisionary work, literature compi lation and expeditions to the Beringian area, 98 species of the Noctuidae are listed as Holarctic and grouped according to their taxonomic and distributional history. Of the 44 species considered to be "naturall y" Holarctic before this study, 27 (61 %) are confirmed as Holarctic; 16 species are added on account of range extensions and 29 because of changes in their taxonomic status; 17 taxa are deleted from the Holarctic list. This brings the total of the group to 72 species. Thirteen species are considered to be introduced by man from Europe, a further eight to have been transported by man in the subtropical areas, and five migrant species, three of them of Neotropical origin, may have been assisted by man. The m~jority of the "naturally" Holarctic species are associated with tundra habitats. The species of dry tundra are frequently endemic to Beringia. In the taiga zone, most Holarctic connections consist of Palaearctic/ Nearctic species pairs. The proportion ofHolarctic species decreases from 100 % in the High Arctic to between 40 and 75 % in Beringia and the northern taiga zone, and from between 10 and 20 % in Newfoundland and Finland to between 2 and 4 % in southern Ontario, Central Europe, Spain and Primorye. -

Check List of Noctuid Moths (Lepidoptera: Noctuidae And
Бiологiчний вiсник МДПУ імені Богдана Хмельницького 6 (2), стор. 87–97, 2016 Biological Bulletin of Bogdan Chmelnitskiy Melitopol State Pedagogical University, 6 (2), pp. 87–97, 2016 ARTICLE UDC 595.786 CHECK LIST OF NOCTUID MOTHS (LEPIDOPTERA: NOCTUIDAE AND EREBIDAE EXCLUDING LYMANTRIINAE AND ARCTIINAE) FROM THE SAUR MOUNTAINS (EAST KAZAKHSTAN AND NORTH-EAST CHINA) A.V. Volynkin1, 2, S.V. Titov3, M. Černila4 1 Altai State University, South Siberian Botanical Garden, Lenina pr. 61, Barnaul, 656049, Russia. E-mail: [email protected] 2 Tomsk State University, Laboratory of Biodiversity and Ecology, Lenina pr. 36, 634050, Tomsk, Russia 3 The Research Centre for Environmental ‘Monitoring’, S. Toraighyrov Pavlodar State University, Lomova str. 64, KZ-140008, Pavlodar, Kazakhstan. E-mail: [email protected] 4 The Slovenian Museum of Natural History, Prešernova 20, SI-1001, Ljubljana, Slovenia. E-mail: [email protected] The paper contains data on the fauna of the Lepidoptera families Erebidae (excluding subfamilies Lymantriinae and Arctiinae) and Noctuidae of the Saur Mountains (East Kazakhstan). The check list includes 216 species. The map of collecting localities is presented. Key words: Lepidoptera, Noctuidae, Erebidae, Asia, Kazakhstan, Saur, fauna. INTRODUCTION The fauna of noctuoid moths (the families Erebidae and Noctuidae) of Kazakhstan is still poorly studied. Only the fauna of West Kazakhstan has been studied satisfactorily (Gorbunov 2011). On the faunas of other parts of the country, only fragmentary data are published (Lederer, 1853; 1855; Aibasov & Zhdanko 1982; Hacker & Peks 1990; Lehmann et al. 1998; Benedek & Bálint 2009; 2013; Korb 2013). In contrast to the West Kazakhstan, the fauna of noctuid moths of East Kazakhstan was studied inadequately. -

Lepidoptera of the Tolman Bridge Area (2000-2011)
LEPIDOPTERA OF THE TOLMAN BRIDGE AREA, ALBERTA, 2000-2011 Charles Bird, 8 March 2012 Box 22, Erskine, AB T0C 1G0 [email protected] The present paper includes a number of redeterminations and additions to the information in earlier reports. It also follows the up-to-date order and taxonomy of Pohl et al. (2010), rather than that of Hodges et al. (1983). Brian Scholtens, Greg Pohl and Jean-François Landry collecting moths at a sheet illuminated by a mercury vapor (MV) light, Tolman Bridge, 24 July 2003, during the 2003 Olds meetings of the Lepidopterist’s Society (C.D. Bird image). Tolman Bridge, is located in the valley of the Red Deer River, 18 km (10 miles) east of the town of Trochu. The bridge and adjoining Park land are in the north half of section 14, range 22, township 34, west of the Fourth Meridian. The coordinates at the bridge are 51.503N and 113.009W. The elevation ranges from around 600 m at the river to 800 m or so near the top of the river breaks. In a Natural Area Inspection Report dated 25 June 1982 and in the 1989 Trochu 82 P/14, 1:50,000 topographic map, the land southwest of the bridge was designated as the “Tolman Bridge Municipal Park” while that southeast of the bridge was referred to as the “Tolman Bridge Recreation Area”. In an Alberta, Department of the Environment, Parks and Protected Areas Division paper dated 9 May 2000, the areas on both sides of the river are included in “Dry Island Buffalo Jump Provincial Park”. -

Moth Diversity in Young Jack Pine-Deciduous Forests Aiter Disturbance by Wildfire Or Clear-Cutting
MOTH DIVERSITY IN YOUNG JACK PINE-DECIDUOUS FORESTS AITER DISTURBANCE BY WILDFIRE OR CLEAR-CUTTING Rosalind Frances Cordes Chaundy A thesis submitted in conformity with the nquirements for the degree of Muter of Science in Foresty Gnduate Faculty of Forest y University of Toronto Q Copyright by Rosrlind Frances Cordu Chiundy 1999 National Library Bibliothbque nationale I*I oi canaci, du Canada Acquisitions and Acquisitions et Bibliogmphic SeMces secvices bibliographiques 395 Wellington Street 395, rue Wellington ûttawaON K1A ON4 Ottawa ON K1A ON4 Canada canada The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant a la National Library of Canada to Bibliothèque nationale du Canada de reproduce, loan, distribute or sel1 reproduire, prêter, distribuer ou copies of this thesis in rnicroform, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfichelfilm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fiom it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. ABSTRACT Moth Diversity in Young Jack Pine-Deciduous Forests Mer Disturbance by Wfldfire or Clear- Cutting. Muter of Science in Forestry. 1999. Roselind Frances Cordes Chaundy. Fadty of Forestry, University of Toronto. Moth divcrsity was compared between four to eight yw-old jack pinedeciduous forests that had ban burned by wildfire or clearîut. -

MOTHS and BUTTERFLIES LEPIDOPTERA DISTRIBUTION DATA SOURCES (LEPIDOPTERA) * Detailed Distributional Information Has Been J.D
MOTHS AND BUTTERFLIES LEPIDOPTERA DISTRIBUTION DATA SOURCES (LEPIDOPTERA) * Detailed distributional information has been J.D. Lafontaine published for only a few groups of Lepidoptera in western Biological Resources Program, Agriculture and Agri-food Canada. Scott (1986) gives good distribution maps for Canada butterflies in North America but these are generalized shade Central Experimental Farm Ottawa, Ontario K1A 0C6 maps that give no detail within the Montane Cordillera Ecozone. A series of memoirs on the Inchworms (family and Geometridae) of Canada by McGuffin (1967, 1972, 1977, 1981, 1987) and Bolte (1990) cover about 3/4 of the Canadian J.T. Troubridge fauna and include dot maps for most species. A long term project on the “Forest Lepidoptera of Canada” resulted in a Pacific Agri-Food Research Centre (Agassiz) four volume series on Lepidoptera that feed on trees in Agriculture and Agri-Food Canada Canada and these also give dot maps for most species Box 1000, Agassiz, B.C. V0M 1A0 (McGugan, 1958; Prentice, 1962, 1963, 1965). Dot maps for three groups of Cutworm Moths (Family Noctuidae): the subfamily Plusiinae (Lafontaine and Poole, 1991), the subfamilies Cuculliinae and Psaphidinae (Poole, 1995), and ABSTRACT the tribe Noctuini (subfamily Noctuinae) (Lafontaine, 1998) have also been published. Most fascicles in The Moths of The Montane Cordillera Ecozone of British Columbia America North of Mexico series (e.g. Ferguson, 1971-72, and southwestern Alberta supports a diverse fauna with over 1978; Franclemont, 1973; Hodges, 1971, 1986; Lafontaine, 2,000 species of butterflies and moths (Order Lepidoptera) 1987; Munroe, 1972-74, 1976; Neunzig, 1986, 1990, 1997) recorded to date.