Emerging Therapies for Non-Small Cell Lung Cancer Chao Zhang1,3, Natasha B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Screening and Identification of Key Biomarkers in Clear Cell Renal Cell Carcinoma Based on Bioinformatics Analysis
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423889; this version posted December 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Screening and identification of key biomarkers in clear cell renal cell carcinoma based on bioinformatics analysis Basavaraj Vastrad1, Chanabasayya Vastrad*2 , Iranna Kotturshetti 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karanataka, India. 3. Department of Ayurveda, Rajiv Gandhi Education Society`s Ayurvedic Medical College, Ron, Karnataka 562209, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423889; this version posted December 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Clear cell renal cell carcinoma (ccRCC) is one of the most common types of malignancy of the urinary system. The pathogenesis and effective diagnosis of ccRCC have become popular topics for research in the previous decade. In the current study, an integrated bioinformatics analysis was performed to identify core genes associated in ccRCC. An expression dataset (GSE105261) was downloaded from the Gene Expression Omnibus database, and included 26 ccRCC and 9 normal kideny samples. Assessment of the microarray dataset led to the recognition of differentially expressed genes (DEGs), which was subsequently used for pathway and gene ontology (GO) enrichment analysis. -

The Impact of Immunological Checkpoint Inhibitors and Targeted Therapy on Chronic Pruritus in Cancer Patients
biomedicines Review The Impact of Immunological Checkpoint Inhibitors and Targeted Therapy on Chronic Pruritus in Cancer Patients Alessandro Allegra 1,* , Eleonora Di Salvo 2 , Marco Casciaro 3,4, Caterina Musolino 1, Giovanni Pioggia 5 and Sebastiano Gangemi 3,4 1 Division of Hematology, Department of Human Pathology in Adulthood and Childhood “Gaetano Barresi”, University of Messina, 98125 Messina, Italy; [email protected] 2 Department of Veterinary Sciences, University of Messina, 98125 Messina, Italy; [email protected] 3 School of Allergy and Clinical Immunology, Department of Clinical and Experimental Medicine, University of Messina, 98125 Messina, Italy; [email protected] (M.C.); [email protected] (S.G.) 4 Operative Unit of Allergy and Clinical Immunology, Department of Clinical and Experimental Medicine, University of Messina, 98125 Messina, Italy 5 Institute for Biomedical Research and Innovation (IRIB), National Research Council of Italy (CNR), 98164 Messina, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-090-221-2364 Abstract: Although pruritus may sometimes be a consequential situation to neoplasms, it more frequently emerges after commencing chemotherapy. In this review, we present our analysis of the chemotherapy treatments that most often induce skin changes and itching. After discussing conventional chemotherapies capable of inducing pruritus, we present our evaluation of new drugs such as immunological checkpoint inhibitors (ICIs), tyrosine kinase inhibitors, and monoclonal antibodies. Although ICIs and targeted therapy are thought to damage tumor cells, these therapies can modify homeostatic events of the epidermis and dermis, causing the occurrence of cutaneous toxicities in treated subjects. In the face of greater efficacy, greater skin toxicity has been reported for most of these drugs. -

MET Or NRAS Amplification Is an Acquired Resistance Mechanism to the Third-Generation EGFR Inhibitor Naquotinib
www.nature.com/scientificreports OPEN MET or NRAS amplifcation is an acquired resistance mechanism to the third-generation EGFR inhibitor Received: 5 October 2017 Accepted: 16 January 2018 naquotinib Published: xx xx xxxx Kiichiro Ninomiya1, Kadoaki Ohashi1,2, Go Makimoto1, Shuta Tomida3, Hisao Higo1, Hiroe Kayatani1, Takashi Ninomiya1, Toshio Kubo4, Eiki Ichihara2, Katsuyuki Hotta5, Masahiro Tabata4, Yoshinobu Maeda1 & Katsuyuki Kiura2 As a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), osimeritnib is the standard treatment for patients with non-small cell lung cancer harboring the EGFR T790M mutation; however, acquired resistance inevitably develops. Therefore, a next-generation treatment strategy is warranted in the osimertinib era. We investigated the mechanism of resistance to a novel EGFR-TKI, naquotinib, with the goal of developing a novel treatment strategy. We established multiple naquotinib-resistant cell lines or osimertinib-resistant cells, two of which were derived from EGFR-TKI-naïve cells; the others were derived from geftinib- or afatinib-resistant cells harboring EGFR T790M. We comprehensively analyzed the RNA kinome sequence, but no universal gene alterations were detected in naquotinib-resistant cells. Neuroblastoma RAS viral oncogene homolog (NRAS) amplifcation was detected in naquotinib-resistant cells derived from geftinib-resistant cells. The combination therapy of MEK inhibitors and naquotinib exhibited a highly benefcial efect in resistant cells with NRAS amplifcation, but the combination of MEK inhibitors and osimertinib had limited efects on naquotinib-resistant cells. Moreover, the combination of MEK inhibitors and naquotinib inhibited the growth of osimertinib-resistant cells, while the combination of MEK inhibitors and osimertinib had little efect on osimertinib-resistant cells. -

Comparative Efficacy and Safety of Lorlatinib and Alectinib for ALK
cancers Systematic Review Comparative Efficacy and Safety of Lorlatinib and Alectinib for ALK-Rearrangement Positive Advanced Non-Small Cell Lung Cancer in Asian and Non-Asian Patients: A Systematic Review and Network Meta-Analysis Koichi Ando 1,2,* , Ryo Manabe 1, Yasunari Kishino 1, Sojiro Kusumoto 1, Toshimitsu Yamaoka 3 , Akihiko Tanaka 1, Tohru Ohmori 1,4 and Hironori Sagara 1 1 Division of Respiratory Medicine and Allergology, Department of Medicine, Showa University School of Medicine, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8666, Japan; [email protected] (R.M.); [email protected] (Y.K.); [email protected] (S.K.); [email protected] (A.T.); [email protected] (T.O.); [email protected] (H.S.) 2 Division of Internal Medicine, Showa University Dental Hospital Medical Clinic, Senzoku Campus, Showa University, 2-1-1 Kita-senzoku, Ohta-ku, Tokyo 145-8515, Japan 3 Advanced Cancer Translational Research Institute, Showa University, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8555, Japan; [email protected] 4 Department of Medicine, Division of Respiratory Medicine, Tokyo Metropolitan Health and Hospitals Corporation, Ebara Hospital, 4-5-10 Higashiyukigaya, Ohta-ku, Tokyo 145-0065, Japan * Correspondence: [email protected]; Tel.: +81-3-3784-8532 Citation: Ando, K.; Manabe, R.; Kishino, Y.; Kusumoto, S.; Yamaoka, Simple Summary: The treatment of anaplastic lymphoma kinase (ALK) rearrangement-positive T.; Tanaka, A.; Ohmori, T.; Sagara, H. (ALK-p) advanced non-small cell lung cancer (NSCLC) remains a challenge. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4,871 Patients Shumei Kato1, Vivek Subbiah2, Erica Marchlik3, Sheryl K
Published OnlineFirst September 28, 2016; DOI: 10.1158/1078-0432.CCR-16-1679 Personalized Medicine and Imaging Clinical Cancer Research RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4,871 Patients Shumei Kato1, Vivek Subbiah2, Erica Marchlik3, Sheryl K. Elkin3, Jennifer L. Carter3, and Razelle Kurzrock1 Abstract Purpose: Aberrations in genetic sequences encoding the tyrosine (52/88)], cell cycle–associated genes [39.8% (35/88)], the PI3K kinase receptor RET lead to oncogenic signaling that is targetable signaling pathway [30.7% (27/88)], MAPK effectors [22.7% with anti-RET multikinase inhibitors. Understanding the compre- (20/88)], or other tyrosine kinase families [21.6% (19/88)]. hensive genomic landscape of RET aberrations across multiple RET fusions were mutually exclusive with MAPK signaling cancers may facilitate clinical trial development targeting RET. pathway alterations. All 72 patients harboring coaberrations Experimental Design: We interrogated the molecular portfolio had distinct genomic portfolios, and most [98.6% (71/72)] of 4,871 patients with diverse malignancies for the presence of had potentially targetable coaberrations with either an FDA- RET aberrations using Clinical Laboratory Improvement Amend- approved or an investigational agent. Two cases with lung ments–certified targeted next-generation sequencing of 182 or (KIF5B-RET) and medullary thyroid carcinoma (RET M918T) 236 gene panels. thatrespondedtoavandetanib(multikinase RET inhibitor)- Results: Among diverse cancers, RET aberrations were iden- containing regimen are shown. tified in 88 cases [1.8% (88/4, 871)], with mutations being Conclusions: RET aberrations were seen in 1.8% of diverse the most common alteration [38.6% (34/88)], followed cancers, with most cases harboring actionable, albeit dis- by fusions [30.7% (27/88), including a novel SQSTM1-RET] tinct, coexisting alterations. -

Combination Immune Therapy
Combination Immune Therapy Translational Medicine Plenary SWOG April 17, 2017 San Francisco T‐cell Activation, Proliferation, and Function is Controlled by Multiple Agonist and Antagonist Signals 1. Co‐stimulation via CD28 ligation 2. CTLA‐4 ligation on activated T 3. T cell function in tissue is subject transduces T cell activating signals cells down‐regulates T cell to feedback inhibition responses T cell Functional Block T cell Activation T cell Activation T cell Proliferative Block T cell T cell APC T cell CTLA‐4 T cell CTLA‐4 TCR TCR PD‐1 PD‐1 PD‐1 IFN‐gamma TCR CD28 Cytokines TCR CD4 MHC MHC PD‐‐L1 MHC B7 MHC CD28 B7 PD‐‐L1 Tumor or Tumor or APC APC Immune cell B7 immune cell APC NK Presence of PD-L1 or TILs1 PPD-L1 /TIL+ PD-L1/TIL + + PD-L1 /TIL+ + PD-L1 /TIL PD-L1 /TIL D-L1 /TIL+ Schalper and Rimm, Yale University NSCLC PD-L1/TIL 45% 17% 26% 12% Type 1 Type 2 Type 3 Type 4 45% 41% 13% 1% Melanoma45% 17% 26% 12% Taube et al Type 1 Type 2 Type 3 Type 4 Tumor‐specific T cells are contained in the PD‐1+ TIL population and are functional after in vitro culture Spectrum of PD‐1/PD‐L1 Antagonist Activity Active • Melanoma Minimal to no activity • Renal cancer (clear cell and non‐clear cell) • Prostate cancer • NSCLC – adenocarcinoma and squamous cell • MMR+ (MSS) colon cancer • Small cell lung cancer • Myeloma • Head and neck cancer • Pancreatic cancer • Gastric and gastroesophageal junction • MMR‐repair deficient tumors (colon, cholangiocarcinoma) • Bladder • Triple negative breast cancer • Ovarian Major PD‐1/PD‐L1 antagonists • Hepatocellular -
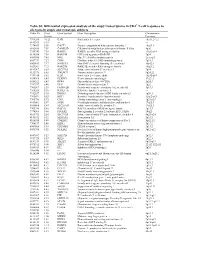
Table S1| Differential Expression Analysis of the Atopy Transcriptome
Table S1| Differential expression analysis of the atopy transcriptome in CD4+ T-cell responses to allergens in atopic and nonatopic subjects Probe ID S.test Gene Symbol Gene Description Chromosome Statistic Location 7994280 10.32 IL4R Interleukin 4 receptor 16p11.2-12.1 8143383 8.95 --- --- --- 7974689 8.50 DACT1 Dapper, antagonist of beta-catenin, homolog 1 14q23.1 8102415 7.59 CAMK2D Calcium/calmodulin-dependent protein kinase II delta 4q26 7950743 7.58 RAB30 RAB30, member RAS oncogene family 11q12-q14 8136580 7.54 RAB19B GTP-binding protein RAB19B 7q34 8043504 7.45 MAL Mal, T-cell differentiation protein 2cen-q13 8087739 7.27 CISH Cytokine inducible SH2-containing protein 3p21.3 8000413 7.17 NSMCE1 Non-SMC element 1 homolog (S. cerevisiae) 16p12.1 8021301 7.15 RAB27B RAB27B, member RAS oncogene family 18q21.2 8143367 6.83 SLC37A3 Solute carrier family 37 member 3 7q34 8152976 6.65 TMEM71 Transmembrane protein 71 8q24.22 7931914 6.56 IL2R Interleukin 2 receptor, alpha 10p15-p14 8014768 6.43 PLXDC1 Plexin domain containing 1 17q21.1 8056222 6.43 DPP4 Dipeptidyl-peptidase 4 (CD26) 2q24.3 7917697 6.40 GFI1 Growth factor independent 1 1p22 7903507 6.39 FAM102B Family with sequence similarity 102, member B 1p13.3 7968236 5.96 RASL11A RAS-like, family 11, member A --- 7912537 5.95 DHRS3 Dehydrogenase/reductase (SDR family) member 3 1p36.1 7963491 5.83 KRT1 Keratin 1 (epidermolytic hyperkeratosis) 12q12-q13 7903786 5.72 CSF1 Colony stimulating factor 1 (macrophage) 1p21-p13 8019061 5.67 SGSH N-sulfoglucosamine sulfohydrolase (sulfamidase) 17q25.3 -

BLU-667 Is a Potent and Highly Selective RET Inhibitor in Development for RET-Driven Thyroid Cancers
BLU-667 is a potent and highly selective RET inhibitor in development for RET-driven thyroid cancers Rami Rahal, PhD Blueprint Medicines July 30, 2017 Disclosure ▪ Employee and shareholder of Blueprint Medicines ▪ BLU-667 is an investigational agent currently in development by Blueprint Medicines 2 REarranged during Transfection (RET) ▪ Receptor tyrosine kinase that transduces signals from GDNF-family ligands ▪ One of the first oncogenic kinase fusions cloned from an epithelial tumor Mulligan, NRC, 2014 NRC, Mulligan, 1987 1990 1993 2012 2013 2014 2015 RET = RTK Papillary Thyroid Medullary Thyroid Lung CMML Colon, Breast, Inflammatory Cancer Cancer (MTC) Adeno Salivary, Myofibroblastic PTC1 = RET Ovarian Tumors Tumors 3 RET Kinase Fusions and Mutations are Oncogenic RET fusions RET mutations Kinase RET + Dimerization domain Fusion Partner ECD M918T V804L/M * * * * * Dimerization domain Kinase RET/PTC Fusion Kinase ▪ ~10% of papillary thyroid cancer ▪ ~60% of medullary thyroid cancer patients (MTC) patients harbor oncogenic ▪ 1-2% of NSCLC patients RET mutations ▪ <1% of patients with colon, ovary, ▪ M918T is the most prevalent RET breast, or hematological cancer mutation 4 Kinase Inhibitors Approved for Treating MTC were Not Designed to Selectively Inhibit RET ▪ Broad kinome activity with potent inhibition of VEGFR-2 ▪ Off-target related dose limiting toxicities hamper ability to inhibit fully RET VEGFR-2 RET Overall Compound Intended Serious adverse Biochem. Biochem. Response (Trade Name) Target(s) events IC50 (nM) IC50 (nM) Rate in MTC Cabozantinib Perforations and VEGFR-2 / MET 2 11 27% (Cometriq) fistulas; hemorrhage QT prolongation; Vandetanib VEGFR-2 / EGFR 4 4 Torsades de pointes; 44% (Calpresa*) sudden death *Only available through Calpresa REMS due to safety concerns 5 BLU-667: a Highly Potent and Selective RET Inhibitor 1. -

The Role of Single Arm Trials in Oncology Drug Development EMA-ESMO Workshop on Single-Arm Trials for Cancer Drug Market Access 30Th June 2016
The role of Single Arm Trials in Oncology Drug Development EMA-ESMO Workshop on single-arm trials for cancer drug market access 30th June 2016 Gideon Blumenthal, MD Office of Hematology and Oncology Products U.S. Food and Drug Administration Challenges with “newparadigm” with Challenges Challenges ROS1 MET PTEN+ PTEN+ Targeted KRAS Therapy N=50 p53 with “oldparadigm” - 200 LKB1 ??? ORR Magnitude, Durable Large EGFR ALK N=800 R - 1200 1. Feasibility of screening/enrolling pts for RCT? for pts screening/enrolling of Feasibility 1. 2. Equipoise Lost? Equipoise 2. Platinum doublet Platinum Platinum doublet Platinum + drug X EFFECT CLINICALLY DETECTION “FAILURE” HIGHRISK 30 ) % ( 25 s n Patients with TARGET Gene events by category (%) o i t 0 25 50 75 100 a r 20 e All t l a Common (5% or greater) e 15 Intermediate (2−5%) n e g Rare (Less than 2%) T 10 2 E G R A 5 T 0 3 1 3 4 5 1 2 4 L 2 1 2 2 3 3 1 1 7 1 1 2 1 2 2 1 1 4 1 1 3 1 1 4 4 2 2 6 1 6 2 1 1 1 4 1 L 4 2 2 2 1 1 2 1 1 1 3 1 3 1 1 3 3 1 2 2 2 2 1 L 2 4 5 L 1 1 1 1 2 3 6 F T T T F F A A S P S A B S K K A B B A S A A A A R N C R R R C R R G O Q M I I 5 L L L 1 L 1 L − F T T T T F F T T P 2 A B A S A A K B B B K 1 K A B X L K V 2 K K P 3 V K E K T S V V P B K 1 A H H R H E H R E D H D R 1 H H C R N D D D R R R H H D D H C H D A OR F T A B A A Y O A K C E P R Y R A M M R A T D W O R I K M L B 2 P X K 2 B 2 2 A 3 C K T E T T L A T T C K K A B N Z F F F F N B B A B A A A S 3 N H H C M C R R N N A C N D S S S E N R A S S N S V C D N A R T C C F D R D C R F N N D R N D R R B O S P R A R N R A -

Targeting ALK: Precision Medicine Takes on Drug Resistance
Published OnlineFirst January 25, 2017; DOI: 10.1158/2159-8290.CD-16-1123 REVIEW Targeting ALK: Precision Medicine Takes on Drug Resistance Jessica J. Lin 1 , Gregory J. Riely 2 , and Alice T. Shaw 1 ABSTRACT Anaplastic lymphoma kinase (ALK) is a validated molecular target in several ALK - rearranged malignancies, including non–small cell lung cancer. However, the clinical benefi t of targeting ALK using tyrosine kinase inhibitors (TKI) is almost universally limited by the emergence of drug resistance. Diverse mechanisms of resistance to ALK TKIs have now been discov- ered, and these basic mechanisms are informing the development of novel therapeutic strategies to overcome resistance in the clinic. In this review, we summarize the current successes and challenges of targeting ALK. Signifi cance: Effective long-term treatment of ALK -rearranged cancers requires a mechanistic under- standing of resistance to ALK TKIs so that rational therapies can be selected to combat resistance. This review underscores the importance of serial biopsies in capturing the dynamic therapeutic vulnerabili- ties within a patient’s tumor and offers a perspective into the complexity of on-target and off-target ALK TKI resistance mechanisms. Therapeutic strategies that can successfully overcome, and poten- tially prevent, these resistance mechanisms will have the greatest impact on patient outcome. Cancer Discov; 7(2); 137–55. ©2017 AACR. INTRODUCTION trials demonstrating remarkable responses in this patient population ( 3–8 ). Yet, as with any targeted therapy, tumor The discovery of anaplastic lymphoma kinase ( ALK ) dates cells evolve and invariably acquire resistance to ALK TKIs, back to 1994 when a chromosomal rearrangement t(2;5), leading to clinical relapse. -

Trastuzumab and Paclitaxel in Patients with EGFR Mutated NSCLC That Express HER2 After Progression on EGFR TKI Treatment
www.nature.com/bjc ARTICLE Clinical Study Trastuzumab and paclitaxel in patients with EGFR mutated NSCLC that express HER2 after progression on EGFR TKI treatment Adrianus J. de Langen1,2, M. Jebbink1, Sayed M. S. Hashemi2, Justine L. Kuiper2, J. de Bruin-Visser2, Kim Monkhorst3, Erik Thunnissen4 and Egbert F. Smit1,2 BACKGROUND: HER2 expression and amplification are observed in ~15% of tumour biopsies from patients with a sensitising EGFR mutation who develop EGFR TKI resistance. It is unknown whether HER2 targeting in this setting can result in tumour responses. METHODS: A single arm phase II study was performed to study the safety and efficacy of trastuzumab and paclitaxel treatment in patients with a sensitising EGFR mutation who show HER2 expression in a tumour biopsy (IHC ≥ 1) after progression on EGFR TKI treatment. Trastuzumab (first dose 4 mg/kg, thereafter 2 mg/kg) and paclitaxel (60 mg/m2) were dosed weekly until disease progression or unacceptable toxicity. The primary end-point was tumour response rate according to RECIST v1.1. RESULTS: Twenty-four patients were enrolled. Nine patients were exon 21 L858R positive and fifteen exon 19 del positive. Median HER2 IHC was 2+ (range 1–3). For 21 patients, gene copy number by in situ hybridisation could be calculated: 5 copies/nucleus (n = 9), 5–10 copies (n = 8), and >10 copies (n = 4). An objective response was observed in 11/24 (46%) patients. Highest response rates were seen for patients with 3+ HER2 IHC (12 patients, ORR 67%) or HER2 copy number ≥10 (4 patients, ORR 100%). Median tumour change in size was 42% decrease (range −100% to +53%).