Irradiation Products on Dwarf Planet Makemake M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHORUS: Let's Go Meet the Dwarf Planets There Are Five in Our Solar
Meet the Dwarf Planet Lyrics: CHORUS: Let’s go meet the dwarf planets There are five in our solar system Let’s go meet the dwarf planets Now I’ll go ahead and list them I’ll name them again in case you missed one There’s Pluto, Ceres, Eris, Makemake and Haumea They haven’t broken free from all the space debris There’s Pluto, Ceres, Eris, Makemake and Haumea They’re smaller than Earth’s moon and they like to roam free I’m the famous Pluto – as many of you know My orbit’s on a different path in the shape of an oval I used to be planet number 9, But I break the rules; I’m one of a kind I take my time orbiting the sun It’s a long, long trip, but I’m having fun! Five moons keep me company On our epic journey Charon’s the biggest, and then there’s Nix Kerberos, Hydra and the last one’s Styx 248 years we travel out Beyond the other planet’s regular rout We hang out in the Kuiper Belt Where the ice debris will never melt CHORUS My name is Ceres, and I’m closest to the sun They found me in the Asteroid Belt in 1801 I’m the only known dwarf planet between Jupiter and Mars They thought I was an asteroid, but I’m too round and large! I’m Eris the biggest dwarf planet, and the slowest one… It takes me 557 years to travel around the sun I have one moon, Dysnomia, to orbit along with me We go way out past the Kuiper Belt, there’s so much more to see! CHORUS My name is Makemake, and everyone thought I was alone But my tiny moon, MK2, has been with me all along It takes 310 years for us to orbit ‘round the sun But out here in the Kuiper Belt… our adventures just begun Hello my name’s Haumea, I’m not round shaped like my friends I rotate fast, every 4 hours, which stretched out both my ends! Namaka and Hi’iaka are my moons, I have just 2 And we live way out past Neptune in the Kuiper Belt it’s true! CHORUS Now you’ve met the dwarf planets, there are 5 of them it’s true But the Solar System is a great big place, with more exploring left to do Keep watching the skies above us with a telescope you look through Because the next person to discover one… could be me or you… . -

Dwarf Planet Ceres
Dwarf Planet Ceres drishtiias.com/printpdf/dwarf-planet-ceres Why in News As per the data collected by NASA’s Dawn spacecraft, dwarf planet Ceres reportedly has salty water underground. Dawn (2007-18) was a mission to the two most massive bodies in the main asteroid belt - Vesta and Ceres. Key Points 1/3 Latest Findings: The scientists have given Ceres the status of an “ocean world” as it has a big reservoir of salty water underneath its frigid surface. This has led to an increased interest of scientists that the dwarf planet was maybe habitable or has the potential to be. Ocean Worlds is a term for ‘Water in the Solar System and Beyond’. The salty water originated in a brine reservoir spread hundreds of miles and about 40 km beneath the surface of the Ceres. Further, there is an evidence that Ceres remains geologically active with cryovolcanism - volcanoes oozing icy material. Instead of molten rock, cryovolcanoes or salty-mud volcanoes release frigid, salty water sometimes mixed with mud. Subsurface Oceans on other Celestial Bodies: Jupiter’s moon Europa, Saturn’s moon Enceladus, Neptune’s moon Triton, and the dwarf planet Pluto. This provides scientists a means to understand the history of the solar system. Ceres: It is the largest object in the asteroid belt between Mars and Jupiter. It was the first member of the asteroid belt to be discovered when Giuseppe Piazzi spotted it in 1801. It is the only dwarf planet located in the inner solar system (includes planets Mercury, Venus, Earth and Mars). Scientists classified it as a dwarf planet in 2006. -

Mass of the Kuiper Belt · 9Th Planet PACS 95.10.Ce · 96.12.De · 96.12.Fe · 96.20.-N · 96.30.-T
Celestial Mechanics and Dynamical Astronomy manuscript No. (will be inserted by the editor) Mass of the Kuiper Belt E. V. Pitjeva · N. P. Pitjev Received: 13 December 2017 / Accepted: 24 August 2018 The final publication ia available at Springer via http://doi.org/10.1007/s10569-018-9853-5 Abstract The Kuiper belt includes tens of thousands of large bodies and millions of smaller objects. The main part of the belt objects is located in the annular zone between 39.4 au and 47.8 au from the Sun, the boundaries correspond to the average distances for orbital resonances 3:2 and 2:1 with the motion of Neptune. One-dimensional, two-dimensional, and discrete rings to model the total gravitational attraction of numerous belt objects are consid- ered. The discrete rotating model most correctly reflects the real interaction of bodies in the Solar system. The masses of the model rings were determined within EPM2017—the new version of ephemerides of planets and the Moon at IAA RAS—by fitting spacecraft ranging observations. The total mass of the Kuiper belt was calculated as the sum of the masses of the 31 largest trans-neptunian objects directly included in the simultaneous integration and the estimated mass of the model of the discrete ring of TNO. The total mass −2 is (1.97 ± 0.30) · 10 m⊕. The gravitational influence of the Kuiper belt on Jupiter, Saturn, Uranus and Neptune exceeds at times the attraction of the hypothetical 9th planet with a mass of ∼ 10 m⊕ at the distances assumed for it. -

Giant Planet / Kuiper Belt Flyby
Giant Planet / Kuiper Belt Flyby Amanda Zangari (SwRI) Tiffany Finley (SwRI) with Cecilia Leung (LPL/SwRI) Simon Porter (SwRI) OPAG: February 23, 2017 Take Away • New Horizons provided scientifically valuable exploration of the Kuiper Belt in the New Frontiers cost cap. • The Kuiper Belt is full of objects with a diverse range of stories that go beyond what we learned from Pluto. • Giant Planet flybys add scientific value to a Kuiper Belt mission • Found preliminary trajectory examples for high interest KBOs-- Haumea, Varuna, 2015 RR245 can be reached via Jupiter AND Saturn, Uranus or Neptune flyby in the 2030s. • To be a candidate New Frontiers mission, a 2 Giant planet+KBO mission must be endorsed by a decadal survey according to current rules. New Horizons Heritage NH Jupiter Encounter planned around Pluto flyby timing, which was dominated by achieving quadruple occultations, “interesting” side up. New Horizons Heritage Pluto flyby took advantage of Ecliptic crossing, enabling access to the cold classical belt (where 2014 MU69 is located). New Horizons Heritage 2014 MU69 discovered while in flight. Targeting was from spacecraft propulsion and took advantage of cold classical population density. Object is small, reddish ~40 km diameter. Saturn’s moons show incredible diversity NASA/JPL As do Uranus and Neptune Some Kuiper Belt Geography Where do we want to go? Getting there- JGA “anytime” New Horizons model: Fast Launch, Jupiter Flyby, Launch window every 11 years McGranaghan et al 2011 Can we go to more than just Jupiter? If so, where, what? New Horizons 2 • 2008 launch using New Horizons flight spares • Proposed Jupiter flyby, equinox flyby of Uranus, and flyby of (47171) 1999 TC36 (now know to be trinary). -
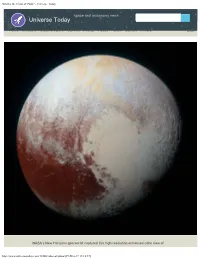
What Is the Color of Pluto? - Universe Today
What is the Color of Pluto? - Universe Today space and astronomy news Universe Today Home Members Guide to Space Carnival Photos Videos Forum Contact Privacy Login NASA’s New Horizons spacecraft captured this high-resolution enhanced color view of http://www.universetoday.com/13866/color-of-pluto/[29-Mar-17 13:18:37] What is the Color of Pluto? - Universe Today Pluto on July 14, 2015. Credit: NASA/JHUAPL/SwRI WHAT IS THE COLOR OF PLUTO? Article Updated: 28 Mar , 2017 by Matt Williams When Pluto was first discovered by Clybe Tombaugh in 1930, astronomers believed that they had found the ninth and outermost planet of the Solar System. In the decades that followed, what little we were able to learn about this distant world was the product of surveys conducted using Earth-based telescopes. Throughout this period, astronomers believed that Pluto was a dirty brown color. In recent years, thanks to improved observations and the New Horizons mission, we have finally managed to obtain a clear picture of what Pluto looks like. In addition to information about its surface features, composition and tenuous atmosphere, much has been learned about Pluto’s appearance. Because of this, we now know that the one-time “ninth planet” of the Solar System is rich and varied in color. Composition: With a mean density of 1.87 g/cm3, Pluto’s composition is differentiated between an icy mantle and a rocky core. The surface is composed of more than 98% nitrogen ice, with traces of methane and carbon monoxide. Scientists also suspect that Pluto’s internal structure is differentiated, with the rocky material having settled into a dense core surrounded by a mantle of water ice. -

The Solar System Cause Impact Craters
ASTRONOMY 161 Introduction to Solar System Astronomy Class 12 Solar System Survey Monday, February 5 Key Concepts (1) The terrestrial planets are made primarily of rock and metal. (2) The Jovian planets are made primarily of hydrogen and helium. (3) Moons (a.k.a. satellites) orbit the planets; some moons are large. (4) Asteroids, meteoroids, comets, and Kuiper Belt objects orbit the Sun. (5) Collision between objects in the Solar System cause impact craters. Family portrait of the Solar System: Mercury, Venus, Earth, Mars, Jupiter, Saturn, Uranus, Neptune, (Eris, Ceres, Pluto): My Very Excellent Mother Just Served Us Nine (Extra Cheese Pizzas). The Solar System: List of Ingredients Ingredient Percent of total mass Sun 99.8% Jupiter 0.1% other planets 0.05% everything else 0.05% The Sun dominates the Solar System Jupiter dominates the planets Object Mass Object Mass 1) Sun 330,000 2) Jupiter 320 10) Ganymede 0.025 3) Saturn 95 11) Titan 0.023 4) Neptune 17 12) Callisto 0.018 5) Uranus 15 13) Io 0.015 6) Earth 1.0 14) Moon 0.012 7) Venus 0.82 15) Europa 0.008 8) Mars 0.11 16) Triton 0.004 9) Mercury 0.055 17) Pluto 0.002 A few words about the Sun. The Sun is a large sphere of gas (mostly H, He – hydrogen and helium). The Sun shines because it is hot (T = 5,800 K). The Sun remains hot because it is powered by fusion of hydrogen to helium (H-bomb). (1) The terrestrial planets are made primarily of rock and metal. -

International Astronomical Union Union Astronomique Internationale
INTERNATIONAL ASTRONOMICAL UNION UNION ASTRONOMIQUE INTERNATIONALE ************ IAU0806: FOR IMMEDIATE RELEASE ************ http://www.iau.org/public_press/news/release/iau0806/ Fourth dwarf planet named Makemake 17 July 2008, Paris: The International Astronomical Union (IAU) has given the name Makemake to the newest member of the family of dwarf planets — the object formerly known as 2005 FY9 —after the Polynesian creator of humanity and the god of fertility. Members of the International Astronomical Union’s Committee on Small Body Nomenclature (CSBN) and the IAU Working Group for Planetary System Nomenclature (WGPSN) have decided to name the newest member of the plutoid family Makemake, and have classified it as the fourth dwarf planet in our Solar System and the third plutoid. Makemake (pronounced MAH-keh MAH-keh) is one of the largest objects known in the outer Solar System and is just slightly smaller and dimmer than Pluto, its fellow plutoid. The dwarf planet is reddish in colour and astronomers believe the surface is covered by a layer of frozen methane. Like other plutoids, Makemake is located in a region beyond Neptune that is populated with small Solar System bodies (often referred to as the transneptunian region). The object was discovered in 2005 by a team from the California Institute of Technology led by Mike Brown and was previously known as 2005 FY9 (or unofficially “Easterbunny”). It has the IAU Minor Planet Center designation (136472). Once the orbit of a small Solar System body or candidate dwarf planet is well determined, its provisional designation (2005 FY9 in the case of Makemake) is superseded by its permanent numerical designation (136472) in the case of Makemake. -

Distant Ekos
Issue No. 51 March 2007 s DISTANT EKO di The Kuiper Belt Electronic Newsletter r Edited by: Joel Wm. Parker [email protected] www.boulder.swri.edu/ekonews CONTENTS News & Announcements ................................. 2 Abstracts of 6 Accepted Papers ......................... 3 Titles of 2 Submitted Papers ........................... 6 Title of 1 Other Paper of Interest ....................... 7 Title of 1 Conference Contribution ..................... 7 Abstracts of 3 Book Chapters ........................... 8 Newsletter Information .............................. 10 1 NEWS & ANNOUNCEMENTS More binaries...lots more... In IAUC 8811, 8814, 8815, and 8816, Noll et al. report satellites of five TNOs from HST observations: • (123509) 2000 WK183, separation = 0.080 arcsec, magnitude difference = 0.4 mag • 2002 WC19, separation = 0.090 arcsec, magnitude difference = 2.5 mag • 2002 GZ31, separation = 0.070 arcsec, magnitude difference = 1.0 mag • 2004 PB108, separation = 0.172 arcsec, magnitude difference = 1.2 mag • (60621) 2000 FE8, separation = 0.044 arcsec, magnitude difference = 0.6 mag In IAUC 8812, Brown and Suer report satellites of four TNOs from HST observations: • (50000) Quaoar, separation = 0.35 arcsec, magnitude difference = 5.6 mag • (55637) 2002 UX25, separation = 0.164 arcsec, magnitude difference = 2.5 mag • (90482) Orcus, separation = 0.25 arcsec, magnitude difference = 2.7 mag • 2003 AZ84, separation = 0.22 arcsec, magnitude difference = 5.0 mag ................................................... ................................................ -

Solar System Planet and Dwarf Planet Fact Sheet
Solar System Planet and Dwarf Planet Fact Sheet The planets and dwarf planets are listed in their order from the Sun. Mercury The smallest planet in the Solar System. The closest planet to the Sun. Revolves the fastest around the Sun. It is 1,000 degrees Fahrenheit hotter on its daytime side than on its night time side. Venus The hottest planet. Average temperature: 864 F. Hotter than your oven at home. It is covered in clouds of sulfuric acid. It rains sulfuric acid on Venus which comes down as virga and does not reach the surface of the planet. Its atmosphere is mostly carbon dioxide (CO2). It has thousands of volcanoes. Most are dormant. But some might be active. Scientists are not sure. It rotates around its axis slower than it revolves around the Sun. That means that its day is longer than its year! This rotation is the slowest in the Solar System. Earth Lots of water! Mountains! Active volcanoes! Hurricanes! Earthquakes! Life! Us! Mars It is sometimes called the "red planet" because it is covered in iron oxide -- a substance that is the same as rust on our planet. It has the highest volcano -- Olympus Mons -- in the Solar System. It is not an active volcano. It has a canyon -- Valles Marineris -- that is as wide as the United States. It once had rivers, lakes and oceans of water. Scientists are trying to find out what happened to all this water and if there ever was (or still is!) life on Mars. It sometimes has dust storms that cover the entire planet. -

Redalyc.Búsqueda General Con La Cámara Mosaico CCD Del
Revista Mexicana de Física ISSN: 0035-001X [email protected] Sociedad Mexicana de Física A.C. México Ferrín, I.; Leal, C.; Hernandez, J. Búsqueda general con la cámara mosaico CCD del telescopio Schmidt de 1m del Observatorio Nacional de Venezuela Revista Mexicana de Física, vol. 52, núm. 3, mayo, 2006, pp. 9-11 Sociedad Mexicana de Física A.C. Distrito Federal, México Disponible en: http://www.redalyc.org/articulo.oa?id=57020393003 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto ASTROFISICA´ REVISTA MEXICANA DE FISICA´ S 52 (3) 9–11 MAYO 2006 Busqueda´ general con la camara´ mosaico CCD del telescopio Schmidt de 1m del Observatorio Nacional de Venezuela I. Ferr´ın y C. Leal Facultad de Ciencias, Departamento de F´ısica, Centro de Astrof´ısica Teorica,´ Universidad de Los Andes Merida,´ Venezuela J. Hernandez Centro de investigaciones de astronom´ıa, CIDA, Merida,´ Venezuela Recibido el 25 de noviembre de 2003; aceptado el 12 de octubre de 2004 En el presente trabajo se muestran los resultados del programa de busqueda´ y seguimiento de diversos objetos astronomicos:´ estrellas variables, supernovas y objetos en movimiento dentro del Sistema Solar. Para ello estamos utilizando el telescopio Schmidt de 1m de diametro´ del Observatorio Nacional de Venezuela, el cual tiene acoplado una camara´ mosaico CCD de 67 Megap´ıxeles, en un arreglo de 4 £ 4 CCDs, cada uno de 2048 £ 2048 p´ıxeles. -

Taxonomy of Trans-Neptunian Objects and Centaurs As Seen from Spectroscopy? F
A&A 604, A86 (2017) Astronomy DOI: 10.1051/0004-6361/201730933 & c ESO 2017 Astrophysics Taxonomy of trans-Neptunian objects and Centaurs as seen from spectroscopy? F. Merlin1, T. Hromakina2, D. Perna1, M. J. Hong1, and A. Alvarez-Candal3 1 LESIA – Observatoire de Paris, PSL Research University, CNRS, Sorbonne Universités, UPMC Univ. Paris 06, Univ. Paris Diderot, Sorbonne Paris Cité, 5 place Jules Janssen, 92195 Meudon, France e-mail: [email protected] 2 Institute of Astronomy, Kharkiv V. N. Karin National University, Sumska Str. 35, 61022 Kharkiv, Ukraine 3 Observatorio Nacional, R. Gal. Jose Cristino 77, 20921-400 Rio de Janeiro, Brazil Received 4 April 2017 / Accepted 19 May 2017 ABSTRACT Context. Taxonomy of trans-Neptunian objects (TNOs) and Centaurs has been made in previous works using broadband filters in the visible and near infrared ranges. This initial investigation led to the establishment of four groups with the aim to provide the mean colors of the different classes with possible links with any physical or chemical properties. However, this taxonomy was only made with the Johnson-Cousins filter system and the ESO J, H, Ks filters combination, and any association with other filter system is not yet available. Aims. We aim to edit complete visible to near infrared taxonomy and extend this work to any possible filters system. To do this, we generate mean spectra for each individual group, from a data set of 43 spectra. This work also presents new spectra of the TNO (38628) Huya, on which aqueous alteration has been suspected, and the Centaur 2007 VH305. -

Artificial Intelligence at the Jet Propulsion Laboratory
AI Magazine Volume 18 Number 1 (1997) (© AAAI) Articles Making an Impact Artificial Intelligence at the Jet Propulsion Laboratory Steve Chien, Dennis DeCoste, Richard Doyle, and Paul Stolorz ■ The National Aeronautics and Space Administra- described here is in the context of the remote- tion (NASA) is being challenged to perform more agent autonomy technology experiment that frequent and intensive space-exploration mis- will fly on the New Millennium Deep Space sions at greatly reduced cost. Nowhere is this One Mission in 1998 (a collaborative effort challenge more acute than among robotic plane- involving JPL and NASA Ames). Many of the tary exploration missions that the Jet Propulsion AI technologists who work at NASA expected Laboratory (JPL) conducts for NASA. This article describes recent and ongoing work on spacecraft to have the opportunity to build an intelli- autonomy and ground systems that builds on a gent spacecraft at some point in their careers; legacy of existing success at JPL applying AI tech- we are surprised and delighted that it has niques to challenging computational problems in come this early. planning and scheduling, real-time monitoring By the year 2000, we expect to demonstrate and control, scientific data analysis, and design NASA spacecraft possessing on-board automat- automation. ed goal-level closed-loop control in the plan- ning and scheduling of activities to achieve mission goals, maneuvering and pointing to execute these activities, and detecting and I research and technology development resolving of faults to continue the mission reached critical mass at the Jet Propul- without requiring ground support. At this Asion Laboratory (JPL) about five years point, mission accomplishment can begin to ago.