G 206 En Instructions for Use
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
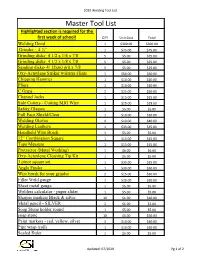
2020 Welding Tool List
2020 Welding Tool List Master Tool List Highlighted section is required for the first week of school! QTY Unit Cost Total Welding Hood 1 $300.00 $300.00 Grinder – 4 ½” 1 $75.00 $75.00 Grinding disks- 4 1/2 x 1/4 x 7/8 5 $5.00 $25.00 Grinding disks- 4 1/2 x 1/8 x 7/8 5 $5.00 $25.00 Sanding disks- 4/ 12x60 grit x 7/8 5 $5.00 $25.00 Oxy-Acteylene Striker w/Extra Flints 1 $50.00 $50.00 Chipping Hammer 1 $10.00 $10.00 Pliers 1 $10.00 $10.00 C Grips 2 $25.00 $50.00 Channel locks 1 $15.00 $15.00 Side Cutters - Cutting MIG Wire 1 $15.00 $15.00 Safety Glasses 1 $5.00 $5.00 Full Face Shield/Clear 1 $10.00 $10.00 Welding Gloves 4 $10.00 $40.00 Welding Leathers 1 $35.00 $35.00 Handheld Wire Brush 1 $5.00 $5.00 12” Combination Square 1 $15.00 $15.00 Tape Measure 1 $15.00 $15.00 Protractor (Metal Working) 1 $6.00 $6.00 Oxy-Acteylene Cleaning Tip Kit 1 $5.00 $5.00 3 piece square set 1 $35.00 $35.00 Angle Finder 1 $30.00 $30.00 Wire brush for your grinder 2 $15.00 $30.00 Fillet Weld gauge 1 $20.00 $20.00 Sheet metal gauge 1 $5.00 $5.00 Welders calculator - paper slider 1 $5.00 $5.00 Sharpie markers Black & silver 10 $1.00 $10.00 Metal pencil - SILVER 2 $1.00 $2.00 Soap Stone holder round 1 $5.00 $5.00 soap stone 10 $5.00 $50.00 Paint markers - red, yellow, silver 3 $10.00 $30.00 Pipe wrap (roll) 1 $10.00 $10.00 Scaled Ruler 1 $5.00 $5.00 Updated: 07/2020 Pg 1 of 2 2020 Welding Tool List Drafting pencil 1 $10.00 $10.00 Graphing Paper 1 $10.00 $10.00 Extra Large Compass (10"-15") 1 $20.00 $20.00 Scribe 1 $50.00 $50.00 Needle nose plyers/3 pc kit 1 $25.00 -

Power Brush Catalog.Pdf
POWER BRUSHES POWER BRUSHES CHOOSE QUALITY Superior construction, the highest quality materials, state of the art manufacturing, and exacting quality standards WHY WEILER deliver the most consistent brush performance. Each Weiler brush is designed to provide the best performance at the lowest cost-of-use. That's why we re-engineered our 4" stringer bead brush to deliver MAXimum impact, letting the wire do the work. Our beefed up Roughneck® Max brush delivers up to BONDED ABRASIVES twice the life. Combine that with the hardest, strongest wire and an improved knot design to MAXimize cleaning power and you have a brush that you can trust when your name is on the line. Technical Information .................................................... 48-56 Knot Wire Wheels ........................................................... 57-63 Weld Cleaning Brushes ................................................. 62-63 Crimped Wire Wheels .................................................... 64-71 COATED ABRASIVES Nylon & Tampico Wheels ...................................................72 Cup Brushes .................................................................... 73-75 Stem-Mounted End Brushes ......................................... 76-79 Crosshole Deburring Brushes ............................................80 Power & Hand Tube Brushes ....................................... 81-85 Crossflex Honing Brushes ............................................. 86-89 Miniature Brushes .......................................................... 90-91 Non-Sparking -

Cutting & Welding Products
WELDING PLASMA OXYFUEL ACCESSORIES Cutting & Welding Products FirepowerOnline.com Advantage Firepower® Products Performance, Value and Ease of Use Firepower, a leader in metal cutting, gas control and specialty welding solutions, is meeting the needs of people who demand professional-quality results, through a refined portfolio of Firepower branded products. These products represent the leading edge in design, performance, quality and safety, backed by 100 years of proven innovation. The Firepower lineup offers superior performance, excellent value and ease-of-use that helps the craftsman to get the job done right the first time. Firepower OxyFuel, incorporating the authentic Victor® torch and regulator range, is the heart of the Firepower family, with system solutions and components designed for individual needs. The OxyFuel range will perform a wide spectrum of tasks, from brazing sheet metal to cutting up to six-inch thick steel. Firepower Plasma offers a broad range of Manual Plasma Cutters capable of slicing up to 1-1/2 inches in multiple metals. These machines are easy to use and can cut a variety of metals, including steel, aluminum, galvanized and more. Firepower MST, with its new line up, comprises the core of the Firepower Welding range. Its technology has changed the game, setting a new standard for portable welding equipment. The 3-in-1 power supplies provide MIG, Stick and TIG capabilities in one integrated system. Firepower Velocity™ consumables allow for easy tip changes (no tip threads, no tools) while delivering longer life. Our consumables are designed for total welding flexibility and optimized welding performance with each process. Firepower draws from the heritage and quality of Victor and Tweco equipment, with the added advantage of utilizing our excellence in product support, service and application know-how. -

Janitorial 326-348
BACK OF HOUSE Clean & Organize www.wincous.com/product-category/back-of-house JA CARPET ENTRYWAY FLOOR MATS ENTRYWAY FLoor MATS ♦♦Vinyl backing prevents curling/ripping and stays flat ♦♦Carts roll easily over the tapered rubber edge NIT ITEM DESCRIPTION COLOR UOM CASE FMC-310C 3' x 10' Charcoal Each 1 FMC-310U 3' x 10' Burgundy Each 1 FMC-35C 3' x 5' Charcoal Each 1 OR FMC-35U 3' x 5' Burgundy Each 1 BACK OF HOUSE FMC-46C 4' x 6' Charcoal Each 1 FMC-46U 4' x 6' Burgundy Each 1 fmC-310C I Clean & Organize A Tapered, fmC-310U weighted, durable L Lays flat & does not curl/rip Tapered, weighted, with constant foot traffic durable edge ANTI-FATIGUE RUBBERIZED GEL FOAM FLOOR MAT ANTI-SLIP & ANTI-FATIGUE ♦♦Ideal for standing-in-place jobs ♦♦Beveled edges reduce tripping FLoor MATS ♦♦Comfortable and supportive closed-cell foam ♦♦Grease-resistant and waterproof ♦♦1/2" thick ITEM DESCRIPTION COLOR UOM CASE FMG-23K 2' x 3' Black Each 24 Beveled edge FMG-23K 1/2" thick STRAIGHT EdGE NATURAL RUBBER FLOOR MATS ♦♦Anti-slip & anti-fatigue top-grade natural rubber ♦♦Excellent drainage in wet areas ♦♦All straight-edge mats are grease-resistant - use in grease-prone areas to prevent slipping ♦♦Trim to customize size for best fit 3/4" thick ♦♦3/4" thick ITEM DESCRIPTION COLOR UOM CASE Grease-resistant RBMH-35K 3' x 5' Black Each 1 RBMH-35R RBMH-35K RBMH-35R 3' x 5' Red Each 1 RBMH-35K-R 3' x 5', Rolled Black Each 1 RBMH-35R-R 3' x 5', Rolled Red Each 1 Available flat BEVELED EDGE FLOOR MATS or rolled ♦♦Anti-slip & anti-fatigue top-grade natural rubber Red -

Automatic Data Processing Equipment Inventory
BASE OPERATING SERVICES (BOS) CONTRACT GOVERNMENT FURNISHED PROPERTY (GFP) INVENTORY 1 - 71 GFP Inventory Report Airfield Management (Building 224) Location Make Model Serial Number GMIAP-440TH BLDG 224 RM 100 Generic table square AF440-001804 GMIAP-440TH BLDG 224 RM 100 Generic table square AF440-001805 GMIAP-440TH BLDG 224 RM 100 Generic bulletin board cork AF440-001806 GMIAP-440TH BLDG 224 RM 100 Generic metal detector walk through AF440-001807 GMIAP-440TH BLDG 224 RM 101 Generic desk metal small AF440-001832 GMIAP-440TH BLDG 224 RM 101 Generic chair straight/conference AF440-001808 GMIAP-440TH BLDG 224 RM 101 Generic chair straight/conference AF440-001809 GMIAP-440TH BLDG 224 RM 101 Generic chair straight/conference AF440-001810 GMIAP-440TH BLDG 224 RM 101 Generic chair straight/conference AF440-001811 GMIAP-440TH BLDG 224 RM 101 Generic chair straight/conference AF440-001812 GMIAP-440TH BLDG 224 RM 101 Generic chair straight/conference AF440-001813 GMIAP-440TH BLDG 224 RM 101 Generic chair straight/conference AF440-001814 GMIAP-440TH BLDG 224 RM 101 Generic chair straight/conference AF440-001815 GMIAP-440TH BLDG 224 RM 101 Generic chair straight/conference AF440-001816 GMIAP-440TH BLDG 224 RM 101 Generic clock wall AF440-001831 GMIAP-440TH BLDG 224 RM 101 Generic poster framed AF440-001828 GMIAP-440TH BLDG 224 RM 101 Generic poster framed AF440-001829 GMIAP-440TH BLDG 224 RM 101 Generic sofa AF440-001817 GMIAP-440TH BLDG 224 RM 101 Generic sofa AF440-001818 GMIAP-440TH BLDG 224 RM 101 Generic table rectangle AF440-001823 GMIAP-440TH -

Glass to Brushes
Do it the HYDE® way at hydetools.com BRUSHES GLASS TOOLS 46850 Glass/Razor Scrapers 13080 Single Edge Blades 13140 Carbon Steel Wire Brush and Blades Utility Blade Glass Scraper Single-edge Razor Blades Stiff carbon steel wire brush with Retractable blade with positive 33255 100-Blade Merchandiser long, curved hardwood handle for Great to keep handy for cleanup lock. Built-in 1/16” sash guide Universal Blades .009" thick. Industrial-quality cleaning jobs where longer reach after painting windows and doors. maintains paint/glass seal. 5 Pack steel. Individually wrapped for Hidden is needed. Brush area measures A ccepts all standard utility knife Versatile 4" replacement blades safety. 20 packs of 5 blades. 1"x6-1/2". Also useful for taking stickers off blades. 1 heavy-duty blade Convenient storage box. included. also snap off to fit 3-1/4" scrap- *Carded, 4.3lb, 6pk mirrors, glass and more. ers. Fits HYDE® scraper #33250 *Boxed, 1.0lb, 1pk *Carded, 1.4lb, 10pk Benefit and other scrapers. Retractable *Carded, 0.7lb, 10pk 13127 No more hunting Mini Brushes Fixed Blade Scrapers Heavy Duty (.012") Razor Blade Scrapers 13120 Single-edge Blades through rusty blades in Single-edge Razor Blades 100 Pack" the cellar! These new 46630 13000 13020 3 Brass Bristle ™ Mini Glass Scraper 5 Pack .012" thick. Industrial-quality ® Delta Scraper HYDE scrapers Mini Brushes plus 1 Blade .009" thick. Industrial-quality steel. Individually wrapped for Rugged polypropylene construc- safety. Reusable plastic container. come with extra Non-spark brass brushes remove tion resists most paint thinners. steel. Individually wrapped Ergonomically engineered to fit for safety. -

REMA TIP TOP Tool Catalogue 2016/2017 Editor
REMA TIP TOP Tool Catalogue 2016/2017 Editor REMA TIP TOP AG Gruber Strasse 65 · 85586 Poing / Germany Phone: +49 8121 707-0 Fax: +49 8121 707-10 222 [email protected] www.rema-tiptop.com Legal Notice Copyright © 2016 REMA TIP TOP AG All information is given to the best of our knowledge. All specifications are to be considered non-binding infor- mation. Any claim for damages of any kind is excluded. We reserve the right to change technical specifications without prior notice, provided that they ensure product improvement. The information presented is based on technical experience but does not guarantee a product´s suitability for specific applications, and does not relieve the users of the responsibility to undertake their own testing, including where any third-party trademark rights are concerned. Pictures in the catalogue may contain optional accessories which are not included in the standard shipment, and need to be ordered additionally. All relevant product information can be found in the respective documents, including operating instructions, technical datasheets as well as conditions for storage and application. The specified qualities of our products are based on information from the accompanying inspections for approval. They represent statistical product data and are not to be considered as guaranteed properties of an individual product. In order to preserve product properties, the storage conditions indicated in DIN 7716 should be followed (including storing the product in the original package and in an area that is dry, cool and -

Technical Information for Wire Brush
Technical01 Information Technical Brush information TECHNICAL INFORMATION The selection of the right type of brush depends on the type of finishing required (fine or coarse) and the characteristics of the surface to be brushed (stainless steel, carbon steel, aluminum, etc.) Also, if the brush is fixed in a machine, it is necessary to know the main features of the machine in order to choose the correct brush type: • The maximum diameter allowed. • The machine’s max RPM. • The arbor hole diameter or the thread type required. Crimped vs. Knotted Wire Wire Diameter The wire gauge of a brush determines the type of surface finishing: • A thicker wire provides a more aggressive brushing action. • A finer wire provides a less aggressive brushing action with a finer finish. Crimped wire brushes, because of their flexibility, are better suited to work on uneven surfaces and they offer a Trim Length more regular and fine finishing. • A shorter trim length makes the brush face rigid and consequently the brush removal capacity is higher. Twist knot wire brushes, offer longer life and a higher removal capacity but offer a rougher finish. • With a longer trim length the brush becomes more flexible, providing a uniform brushing even on irregular surfaces. Wire Brushes vs. Hard Abrasives Wire brushes act as an impact tool removing oxide, paint or other adherence from the surface without damaging the base material, as opposed to hard abrasives which will remove the base material along with the surface adherence. Wire brushes are much more flexible, which allow the end user to clean areas difficult to reach and adapt to irregular surfaces, which bonded and coated abrasives cannot. -

Wire Brush Master
Wire Brush Products Premium Performance Wire Brushes Since 1948 Lessmann wire brushes have been produced in Oettigen Germany. All Lessmann brushes are designed to offer outstanding performance, service life, safety and operator comfort. Thanks to their constant investment in the latest technologies and product innovation Lessmann wire brushes are recognized as premium quality products around the world. Quality guarantee: Lessmann brushes are manufactured according to the existing standard DIN 68 347 part 1 and 2 and EN1083. All the relevant machine driven brushes are checked for occupational safety using a centrifugal force test. Brush Selection Chart Type of Angle of Type of Brush Application Power Tool Use Recommended Angle Grinder Flat Brushing Crimped Wire Cup Brush Light surface cleaning of large surfaces Twist Knot Wire Cup Brush More aggressive brushing and cleaning Angle Grinder Angled Brushing Knot Conical Brushes Working at angles in corners, contours and other hard to reach areas Angle Grinder Vertical Brushing Knot Wire Wheel Brushes Heavy duty surface cleaning Pipeline/Stinger and/or Rose Extremely narrow areas such Bud Wire Brushes as welding seams, providing maximum performance Bench/Pedestal Vertical Brushing Crimped Wheel Brush General purpose cleaning and Grinders deburring Drill Vertical Brushing Crimped Wheel Brush with General purpose cleaning Shank (rust, paint…etc) Twist Knotted Wheel Brush Heavy duty applications with Shank 2 VANGUARD STEEL LTD. Wire Brush Products Hub/Brush color coding Alloyed and manganese wire which offers increased toughness, resulting in higher tensile strength and a longer service life. Premium quality 302/304 stainless steel wire offering a long service life, also resistant against corrosion and high temperatures. -

1 COURSE DESCRIPTION MFGT 61 – Welding 2 (Intermediate
REEDLEY COLLEGE Manufacturing Technology MFGT 61 –Welding 2 (Sch .# 50386) Rm.# Ind. 11&19 --- M-Th 1:00-5:15pm --- Spr 2010 9 week class --- Jan 11 – Mar 11 INSTRUCTOR: Robert Fransen Industrial Technology Building – Welding Department Office: Room 22 Phone: 638-3641, ext. 3253 E-mail: [email protected] Office Hours: M-Th -12:30pm F -11:00 am COURSE DESCRIPTION MFGT 61 – Welding 2 (Intermediate Welding) 4 Units 1 Lecture and 8 Lab Hours per week Prerequisites: MFGT 60 This course is a continuation of basic welding. Emphasis is placed on out-of-position work (horizontal, vertical and overhead.) Processes include Stick, Mig, Flux core, Tig, and cutting processes. 1. Advanced shop welding in MIG and Innershield welding on mild steel, stainless steel and aluminum. Welding will be done in all positions. 2. Advanced shop welding in Tig on mild steel, stainless steel and aluminum. 3. Advance shop welding practices in electric arc welding (SMAW) on mild steel plate and pipe. Welding will be done in flat, horizontal, vertical and overhead position with emphasis on working towards AWS plate certification. 4. Advance shop oxy-acetylene cutting practice using hand torch, straight line cutter, optic cutter and plasma cutting equipment. 5. Student will fill out a job application and review basic job interview skills. EXPECTED OUTCOMES 1. Students will be able to select and use the proper tools correctly as needed in the Students will be able to select and use the proper tools correctly as needed in the welding field. 2. Students will be able to select, adjust and use properly the welding wire, rods and welding power sources. -

LINEMAN INNOVATION Fixed Blade Skinning Knife
THE HOME OF LINEMAN INNOVATION Fixed Blade Skinning Knife Decrease outages, minimize open neutrals and bad connections, & create significant savings by brushing conductors properly! Highest grade stainless steel for THE MOST INNOVATIVE TOOL maximum durability and sharpness JUST GOT BETTER... MORE DURABILITY Pointed angled blade design for maximum sharpness Blade and snap ring are molded for superior strength and separated by nonconductive material Ergonomic design with over mold MAXIMUM SAFETY for secure grip Blunt edges and tip + Finger guard for maximum safety Catalog #: FBSK-1 Cutting area Universal / Shotgun Knife Integrated stainless steel wire brush for Blade can be closed Works in all shot gun sticks and universal sticks cleaning conductors to make better when not in use connections Highest grade stainless steel for blade for maximum durability for the toughest insulation Locks in open position to prevent accidental closing Pointed angled blade design for maximum sharpness Brushes up to 1000 MCM Conductor Replaceable blade (As pictured from top to bottom) Replaceable Base made out of aluminum Catalog #: BB-2 (BrushBlade II) brushes Catalog #: BB-2S (BrushBlade Safety) Catalog #: USSK-1 (Shotgun Knife) Catalog #: WB-1 (Wire Brush Replacement) Catalog #: USRB-1 (Replaceable Blade) SKINNING KNIVES SKINNING KNIVES Mini FlatRound Brush Integrated stainless steel wire brushes for Features of both the cleaning conductors to make better connections DogBone™ and FlatRound™: 2 Tools in 1 Integrated stainless steel wire brushes for clean- -

H14 Pliers USA.Indd 223 13-04-17 11:18 COMBINATION PLIERS 6” LONG NOSE PLIERS 6” DIAGONAL PLIERS 5”
Pliers, hammers, chisels, files, measuring and cutting tools ERGONOMICALLY DESIGNED TWO COMPONENT HANDLE PRECISION-HARDENED PLIERS HEAD FOR ON-THE-JOB TOUGHNESS SONIC PLIERS FEATURE AN ERGONOMIC DESIGN TO GET THE JOB DONE QUICKLY, EFFICIENTLY AND COMFORTABLY. QUALITY FORGED STEEL ENSURES STRENGTH AND TOUGHNESS. HANDLES ARE DESIGNED TO INCREASE LEAVERAGE AND STRENGTH WITHOUT SACRIFICING THE USER COMFORT. DIAGONAL COMBINATION LONG NOSE BENT LONG NOSE SNAP RING PLIERS SNAP RING PLIERS SNAP RING SNAP RING PLIERS PLIERS PLIERS PLIERS STRAIGHT-OPEN STRAIGHT-CLOSE PLIERS BENT-OPEN PLIERS BENT-CLOSE Hammers Grip chisel 14 SHEATED, SHOCK - NEW ERGONOMIC ABSORBING HANDLE, WITH PRECISION HEAT COMFORT GRIP HANDLE PERFECT GRIP. IT OFFERS TREATMENT OF THE HEAD MADE OF 65% FIBREGLASS MAXIMUM COMFORT AND SAFETY AND HIGH QUALITY FACE FINISH COMBINED TO ENHANCE SAFETY FULL RANGE OF SONIC CHISELS SONIC OFFERS YOU THE LATEST FIBERGLASS HAMMERS WITH ERGONOMIC TPR COVER. SEMI SPHERICAL STRIKING AREA CONTROLS MUSHROOMING. PRODUCED FROM SPECIAL SONIC HAS MACHINIST HAMMERS FROM 200GR, 400GR AND 1000GR STONING HAMMER. ALLOY STEEL WITH DIFFERENT HEAT TREATMENT PROCESS TO HELP TOUGHNESS. THE ERGONOMIC SOFT GRIP HANDLE PROVIDES A POWERFUL GRIP, IMPROVES THE THERMICAL SENSATION IN COLD WORK ENVIRONMENTS AND REDUCES STRIKE VIBRATION. SONICTOOLSUSA.COM | 223 H14 pliers USA.indd 223 13-04-17 11:18 COMBINATION PLIERS 6” LONG NOSE PLIERS 6” DIAGONAL PLIERS 5” ART. NO. L(MM) G PCS/BAG ART. NO. L(MM) G PCS/BAG ART. NO. L(MM) G PCS/BAG 4311165 165 190 6 4322160 170 152 6 4341145 155 158 6 COMBINATION PLIERS 7” LONG NOSE PLIERS 6” DIAGONAL PLIERS 7” ART.