Annual Report 2018-19 Annual Report 2018-19
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Operation Theatre Technician Anurag
OPERATION THEATRE TECHNICIAN ANURAG. A. S ADARSH. A. S ANU BHAVAN VIDYA VIHAR, VEERALAM VALIYAPARAMBU ATTINGAL PO MARAYAMUTTOM PO TRIVANDRUM 695101 TRIVANDRUM 695124 Roll No. 1001 Roll No. 1008 AJULAL. T ANURAJ. S. R LINUBHAVANAM, THAZHAM VARUVILA PUTHEN VEEDU EAST KALLADA PO, MAMKOOTTAM THIRUPURAM PO KOLLAM, 691502 TRIVANDRUM 695133 Roll No. 1002 Roll No. 1009 AMAL NATH. R. M ANUSREE. A SARALAMANDIRAM SREELAKAM MADATHUVATHUKKAL KAROOR, POTHENCODE PO MITHIRMALA PO‐ 695610 TRIVANDRUM 695584 Roll No. 1003 Roll No. 1010 ANILA. P. V ARCHANA. S PUTHIYA VEETTIL ARCHANA BHAVAN POOKODE PO PULLOORKONAM, VIZHINJAM PO KANNUR 670691 TRIVANDRUM. 695521 Roll No. 1004 Roll No. 1011 ANTO VINCENT. T ARCHANA. S ANTO NIVAS SREEPADMAM, LMSRA‐65 VIRALY, UCHAKKADA PO L. M. S. JUNCTION, ATTINGAL NEYYATTINKARA TRIVANDRUM, 695101 TRIVANDRUM,695506 Roll No. 1012 Roll No. 1005 ARUN. G. J ANUJA. S. S AMBADI, VAMANAPURAM ADITHYA BHAVAN VAMANAPURAM PO KATTACHAL KUZHI PO TRIVANDRUM, 695606 BALARAMAPURAM Roll No. 1013 TRIVANDRUM 695501 Roll No. 1006 ASWANI. R LAKSHMIGOVINDAM' ANURADHA. S. P KAYAKKAL HOUSE, PAYAMBRA POST SAFALYA KUNNAMANGALAM (VIA) PUTHENVEEDU KOZHIKODE 673571 PALLIPPURAM PO Roll No. 1014 TRIVANDRUM 695316 Roll No. 1007 ASWATHI. S ILANGO. K KRISHNA VILASOM NO. 18, K. K. ILLAM KADATHOOR, K. S. PURAM PO KALIAMMAN KOVIL STREET KARUNAGAPPALLY KEEZHAKASAKUDY, KOTTUCHERRY KOLLAM 690544 KARAIKAL 609609 Roll No. 1015 Roll No. 1023 BABU. T. N JEENA. J. V NO. 20, CONTRACTOR SUBRAMANI FIRST STREET THEKKEYIDA VILAKATHU THORAPADI, VELLORE MELE PUTHEN VEEDU TAMIL NADU, 632002 AMOTTUKONAM, CHAIKOTTUKONAM PO Roll No. 1016 TRIVANDRUM 695122 Roll No. 1024 BIFIN. B ARUMALOORKONAM JINI. M. G MEKKE PLANKALA VEEDU SOUPARNIKA KUNNATHUKAL, KARAKONAM PO MAMPALLIKUNNAM TRIVANDRUM 695504 CHATHANNUR PO Roll No. -

Abstract of the Agenda for the Meeting of Regional
ABSTRACT OF THE AGENDA FOR THE MEETING OF REGIONAL TRANSPORT AUTHORITY MUVATTUPUZHA PROPOSED TO BE HELD ON 06-07-2019 AT 11.00 AM AT PLANNING CONFERENCE HALL, GROUND FLOOR,CIVILSTATION KAKKANAD-ERNAKULAM. Item No. 01 G/1628/2019/EM Agenda:- To consider the application for fresh regular permit in respect of new or suitable stage carriage with seating capacity not less than 28 in all to operate on the route West Morakkala- Aluva (Via) Kizhakambalam, Pukkattupady as ordinary service . Applicant: Sri T M Pareekunju, , Thanisserry House, Kumarapuram P O. Ref:- Decision of RTA Muvattupuzha held on 23/03/2019 on item No-06 Proposed timings. West Morakkla Morakkala Kizhakambalam Aluva A D A D A D A D 6.20 6.15 am 6.59 7.09P 7.43 8.40 8.30P 7.56 9.00 9.10P 9.44 10.38 10.28P 9.54 11.52 12.02P 12.36 1.30 1.24P 12.50 2.05 2.11P 2.45 4.11 4.01P 3.27 5.19 5.29P 6.03 7.01 6.51P 6.17 7.30 7.35 (Halt) Item No. 02 G/29523/2019/EM Agenda:- To consider the application for fresh regular permit in respect of S/c KL 40 Q 9053 or suitable stage carriage with seating capacity not less than 38 in all to operate on the route Anand Oil Mill- Perumbavoor- Aluva touching Kothamangalam (Via) Allapara, Vazhakulam , Odackaly , Kuruppampady as ordinary moffusil service . Applicant: Sri Sulfikker K U, Kokkadan House, Kandantharra, Allapra P O, Perumbavoor . -

Payment Locations - Muthoot
Payment Locations - Muthoot District Region Br.Code Branch Name Branch Address Branch Town Name Postel Code Branch Contact Number Royale Arcade Building, Kochalummoodu, ALLEPPEY KOZHENCHERY 4365 Kochalummoodu Mavelikkara 690570 +91-479-2358277 Kallimel P.O, Mavelikkara, Alappuzha District S. Devi building, kizhakkenada, puliyoor p.o, ALLEPPEY THIRUVALLA 4180 PULIYOOR chenganur, alappuzha dist, pin – 689510, CHENGANUR 689510 0479-2464433 kerala Kizhakkethalekal Building, Opp.Malankkara CHENGANNUR - ALLEPPEY THIRUVALLA 3777 Catholic Church, Mc Road,Chengannur, CHENGANNUR - HOSPITAL ROAD 689121 0479-2457077 HOSPITAL ROAD Alleppey Dist, Pin Code - 689121 Muthoot Finance Ltd, Akeril Puthenparambil ALLEPPEY THIRUVALLA 2672 MELPADAM MELPADAM 689627 479-2318545 Building ;Melpadam;Pincode- 689627 Kochumadam Building,Near Ksrtc Bus Stand, ALLEPPEY THIRUVALLA 2219 MAVELIKARA KSRTC MAVELIKARA KSRTC 689101 0469-2342656 Mavelikara-6890101 Thattarethu Buldg,Karakkad P.O,Chengannur, ALLEPPEY THIRUVALLA 1837 KARAKKAD KARAKKAD 689504 0479-2422687 Pin-689504 Kalluvilayil Bulg, Ennakkad P.O Alleppy,Pin- ALLEPPEY THIRUVALLA 1481 ENNAKKAD ENNAKKAD 689624 0479-2466886 689624 Himagiri Complex,Kallumala,Thekke Junction, ALLEPPEY THIRUVALLA 1228 KALLUMALA KALLUMALA 690101 0479-2344449 Mavelikkara-690101 CHERUKOLE Anugraha Complex, Near Subhananda ALLEPPEY THIRUVALLA 846 CHERUKOLE MAVELIKARA 690104 04793295897 MAVELIKARA Ashramam, Cherukole,Mavelikara, 690104 Oondamparampil O V Chacko Memorial ALLEPPEY THIRUVALLA 668 THIRUVANVANDOOR THIRUVANVANDOOR 689109 0479-2429349 -

NATIONAL MEANS CUM MERIT SCHOLARSHIP EXAMINATION (NMMSE)-2019 (FINAL LIST of ELIGIBLE CANDIDATES) THIRUVANANTHAPURAM DISTRICT GENERAL CATEGORY Sl
NATIONAL MEANS CUM MERIT SCHOLARSHIP EXAMINATION (NMMSE)-2019 (FINAL LIST OF ELIGIBLE CANDIDATES) THIRUVANANTHAPURAM DISTRICT GENERAL CATEGORY Sl. Caste ROLL NO Applicant Name School_Name No Category 1 42192790174 SREEHARI VINOD General Govt. Model HSS For Boys Attingal , Attingal 2 42192830290 GOPIKA I G General Govt. V.H.S.S. Kallara , KALLARA 3 42192730328 ARATHY M General GOVT. H S S, NEDUVELI, KONCHIRA, VEMBAYAM , 4 42192750125 ANAND SWAROOP J S General Govt. H S S Elampa , Elampa 5 42192740003 AMAL A L General L. V. H. S. Pothencode , Pothencode 6 42192860293 DEVANARAYANAN S R General P. P. M. H. S. Karakonam , karakonam 7 42192810350 KRIPA SUDISH General R R V GHSS Kilimanoor , kilimanoor 8 42192830280 ASNA S General Govt. V.H.S.S. Kallara , KALLARA 9 42192870029 AKHILA S General St. Thomas H. S. S. Amboori , Amboori 10 42192830299 MIDHUNA S NAIR General Govt. V.H.S.S. Kallara , KALLARA 11 42192740032 RESHMA S R General St. Goretti's Girls H. S. S. Nalanchira , Nalanchira 12 42192760120 ASHTAMI A S General DBHS Vamanapuram , vamanapuram 13 42192790241 GANGA G PRASANNAN General Govt H S S For Girls Attingal , Attingal 14 42192790227 ATHIDI ANILKUMAR General Govt H S S For Girls Attingal , Attingal 15 42192810135 DEVIKA B General Govt. HSS Kilimanoor , kilimanoor 16 42192820005 ADWAITH S R General Govt V H S S Njekkad , NJEKKAD 17 42192830371 RISHAV RAJ General M R M K M M H S S Edava , EDAVA 18 42192850125 SHANU S General St. Mary`s H. S. S. Vizhinjam , Vizhinjam 19 42192840321 JAYASREE J S General New H. S. -

Reg No: Name Reg.No Reg Date 455188 MONICA CHRISTOPHER 77001 14--07--2020 412394 ARYA.V 77003 14--07--2020 328998 Aswathysasidha
List - XXII Permanent Registration Number alone Allotted (Continuation from List XXI) Details of Permanent Register number allotted to Modern medicine Doctors (Applications received up to 20/08/2020) Certificate will be issued after completing all formal procedures Reg No: Name Name of College` Reg.No Reg Date 455188 MONICA CHRISTOPHER TRAVANCORE MEDICAL COLLEGE, MEDICITY, KOLLAM 77001 14--07--2020 412394 ARYA.V JUBILEE MISSION MEDICAL COLLEGE & RESEARCH INSTITUTE, THRISSUR 77003 14--07--2020 328998 AswathySasidharan Government Medical College, Thrissur 77005 14--07--2020 336170 Anjumol T.A Government Medical College, Kottayam 77006 14--07--2020 463840 SHAMSHEEDA GOVERNMENT MEDICAL COLLEGE , KANNUR 77751 18--08--2020 512297 AKSHAY M DILEEP Government Medical College, Kottayam 77752 18--08--2020 506767 NOUREEN FAZAL Government Medical College, Thrissur 77753 18--08--2020 508435 FIDHA FATHIMA SADAR Government Medical College, Kottayam 77754 18--08--2020 505802 NAHJA ROSHIN A.M. TRAVANCORE MEDICAL COLLEGE, MEDICITY, KOLLAM 77756 18--08--2020 481395 AISWARYA VIJAY V J SREE NARAYANA INSTITUTE OF MEDICAL SCIENCES, ERNAKULAM 77757 18--08--2020 506776 SONA MARIA PETER PK DAS INSTITUTE OF MEDICAL SCIENCES, PALAKKAD 77758 18--08--2020 445548 SWABEEHA ABOOBACKER PK DAS INSTITUTE OF MEDICAL SCIENCES, PALAKKAD 77759 18--08--2020 507536 DHEERAJ T. K. D.M. Wayanad Institute of Medical Sciences, Wayanad 77760 18--08--2020 499438 MURSHIDA P MALABAR MEDICAL COLLEGE HOSPITAL AND RESEARCH CENTER, KOZHIKODE 77761 18--08--2020 383612 Bini Cherian JUBILEE MISSION -
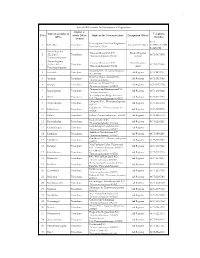
List of Offices Under the Department of Registration
1 List of Offices under the Department of Registration District in Name& Location of Telephone Sl No which Office Address for Communication Designated Officer Office Number located 0471- O/o Inspector General of Registration, 1 IGR office Trivandrum Administrative officer 2472110/247211 Vanchiyoor, Tvpm 8/2474782 District Registrar Transport Bhavan,Fort P.O District Registrar 2 (GL)Office, Trivandrum 0471-2471868 Thiruvananthapuram-695023 General Thiruvananthapuram District Registrar Transport Bhavan,Fort P.O District Registrar 3 (Audit) Office, Trivandrum 0471-2471869 Thiruvananthapuram-695024 Audit Thiruvananthapuram Amaravila P.O , Thiruvananthapuram 4 Amaravila Trivandrum Sub Registrar 0471-2234399 Pin -695122 Near Post Office, Aryanad P.O., 5 Aryanadu Trivandrum Sub Registrar 0472-2851940 Thiruvananthapuram Kacherry Jn., Attingal P.O. , 6 Attingal Trivandrum Sub Registrar 0470-2623320 Thiruvananthapuram- 695101 Thenpamuttam,BalaramapuramP.O., 7 Balaramapuram Trivandrum Sub Registrar 0471-2403022 Thiruvananthapuram Near Killippalam Bridge, Karamana 8 Chalai Trivandrum Sub Registrar 0471-2345473 P.O. Thiruvananthapuram -695002 Chirayinkil P.O., Thiruvananthapuram - 9 Chirayinkeezhu Trivandrum Sub Registrar 0470-2645060 695304 Kadakkavoor, Thiruvananthapuram - 10 Kadakkavoor Trivandrum Sub Registrar 0470-2658570 695306 11 Kallara Trivandrum Kallara, Thiruvananthapuram -695608 Sub Registrar 0472-2860140 Kanjiramkulam P.O., 12 Kanjiramkulam Trivandrum Sub Registrar 0471-2264143 Thiruvananthapuram- 695524 Kanyakulangara,Vembayam P.O. 13 -

Diocese of Kandanad East
THE MALANKARA SYRIAN CHRISTIAN ASSOCIATION List of Members 2017- 2022 Diocese : KANDAND EAST Sl. Name of the Name & Address of the Age Remarks No. Parish Church representatives KND(E)-01/01 01 Aaroor St. Mary’s Rev. Fr. Elias Cherukattu 48 Marygiri Ezhakkaranadu P.O., Puthencruz, Ernakulam Dt. 682308. Mob: 9447820111 KND(E)-01/02 Sri. P.C. Skaria 56 Thakadiyil House, Meenkunnam P.O., Muvattupuzha 686672. Mob: 9847275436 KND(E)-02/01 02 Amayapra St. Mary’s Rev.Fr. Vinod George 44 St. Thomas Dayara, Vettickal, Mulanthruthy, Ernakulam. Mob: 7025168747 KND(E)-02/02 Sri. K.P. Biju 44 Kalledumbil House, Vannappuram P.O., Odiyappara, Thodupuzha, Idukki. Mob: 9495381889 03 Brahmamangalam St. George’s KND(E)-04/01 04 Chempu St. Thomas Rev.Fr. Dr. John Erniyakulathil 56 Erniyakulathil House, Elanji P.O., Kottayam 686665. Mob: 9446122869 KND(E)-04/02 Sri. Animon Chandy 58 Eeraly House, Vaikom West P.O., Vaikom FINALKottayam 686665. LIST KND(E)-05/01 05 Edayar St. Mary’s Rev. Fr. Paulose Skaria 65 Elaprayil House, Oliyappuram P.O., Koothattukulam 686662. Mob: 9446138159 KND(E)-05/02 Sri. George K.J. Kaniyalil Puthenpurayil, Koothattukulam P.O. 686662. Mob: 9447302819 2 KND(E)-06/01 06 Idukki St. Mary’s Rev.Fr. Aby P.U. 33 Parachalil House, Thattakuzhi P.O., Thdupuzha, Idukki 685581. Mob: 9744628565 KND(E)-06/02 Sri. Babu K.K. 52 Kannamkara House, Cheruthoni, Idukki Colony P.O., Idukki 685502. Mob: 9495428766 07 Kalamboor St. George’s KND(E)-08/01 08 Kanniattunirappu Rev. Fr. John Moolamattom 50 St. John’s Vicar, St. -

Academic Counsellors: E-Mail & Mobile Numbers RC 14
ACD_CD ACD_NM ACD_ADDRESS EMAILID ACD_MOBILE RC_CD SC_CD COURSE MADHU VILLA POOZHIKOL P.O. DEVMT:BHC12, DEVMT:BHC13, DEVMT:BHC14, 141172960 RADHIKA P C [email protected] 9995882584 14 1402 KADUTHURUTHY,KOTTAYAM-682013 DEVMT:BHCL11, DEVMT:BHCP11 KOTTAKKAL PUTHENPURA MADOM MAH:MHI1, MAH:MHI2, MAH:MHI3, MAH:MHI4, 141116960 B.SHYAMA 9495368688 14 14000 KOONAMMZVU P.O ERNAKULAM-683518 MAH:MHI5, MAH:MHI8 CHAKKACHAM 141116720 Ms.SHINY K.A VEEDU,KOKOTHAMANGALAM P.O 9446000957 14 14180D CHBHC:CNSHC1, CHBHC:CNSHCP1 CHERTHALA ALAPPUZHA-688527 Kunnanchathanattu House Poochakal Via 1463610 Vijay Kumar K 9895814131 14 14157 BA:BSHF101, MP:MS3, MP:MS8, MP:MS9 Cherthala ARANHIKKAL HOUSE NEAR MUTTOM JUMA 14243400 A.ABOOBACKER MASJID THAIKKATTUKARA P.O. ALUVA 9447174245 14 1400 CAL:BAL1, CAL:BAL2 683106 33/3198 AZHIKKAKATH HOUSE 1464502 A.C.JOSEPH 9447414291 14 14159 DFPT:BPVI42, DFPT:BPVI43 MANAIKKAPARAMB VENNALA 682029 1425369 A.J.JOSE NEWMAN COLLEGE THODUPUZHA 685585 14 1408 MEG:MEG10, MEG:MEG11, MEG:MEG3 P S M O COLLEGE TIRURANGADI P O 1422580 ABDUL AZEEZ M 9847191079 14 1409 BA:EEC11, BA:EEC19 MALAPPURAM 676306 PSMO COLLEGE TIRURANGADI PO 14388840 ABDUL BARI C 14 1409 CFE:BEG4, CFE:BEG5, CFE:BEG6 MALAPPURAM PSMO COLLEGE, TIRURANGADI PO, BA:BEGE101, BA:BEGE102, BA:BEGE103, BA:BEGE104, 14339160 ABDUL BARI C 14 1409 MALAPPURAM BA:BEGE105 CHALIYATHODIKA HOUSE KATTUMUNDA 1442820 ABDUL GAFAR C 9388827390 14 14122 BA:BEGE105, BA:EEG3, BA:EEG4 P.O. NADUVATH MALAPPURAM P S M O COLLEGE TIRURANGADI P O 1422484 ABDUL KARIM C 9847961831 14 1409 BCOM:ECO1, BCOM:ECO13, BCOM:ECO3 MALAPPURAM 676306 PSMO COLLEGE, TIRURANGADI PO, 14339100 ABDUL NASAR V 14 1409 BCOM:ECO10, BCOM:ECO14 MALAPPURAM PSMO COLLEGE TIRURANGADI PO 14339280 ABDUL SAMAD 14 1409 BA:BEGE101, BA:BEGE102, BA:FEG1, BA:FEG2 MALAPPURAM THODUMMANI KIZHETHIL HOUSE MSCCFT:MCFT4, MSCCFT:MCFTL8, MSCCFT:MCFTP1, 1435240 ABDUL SHUKOOR TK PERINTHATTIRI P.O. -
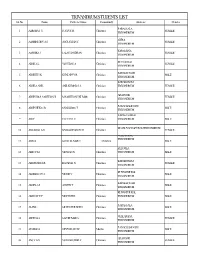
Thiruvananthapuram
TRIVANDRUM STUDENTS LIST SL No Name Father's Name Community Address Gender PARASSALA 1 AARSHA J U JUSTUS H Christian FEMALE TRIVANDRUM AYIRA 2 AASBINI DEV A S AUGUSTIAN C Christian FEMALE TRIVANDRUM KARAMANA 3 AASHIKA J JALACHANDRAN Christian FEMALE TRIVANDRUM KOTTACKAL 4 ABHIJA L YESUDAS A Christian FEMALE TRIVANDRUM KARAKKONAM 5 ABHIJITH K KUNJAPPY R Christian MALE TRIVANDRUM KARAKONAM 6 ABHILA ANIL ANIL KUMAR S S Christian TRIVANDRUM FEMALE ARAYOOR 7 ABHISHA S SANTHOSH M SANTHOSH KUMAR Christian FEMALE TRIVANDRUM PANACHAMOODU 8 ABHISHEK G B GNANADAS T Christian MALE TRIVANDRUM PARASUVAIKAL 9 ABI C CLEETUS C Christian TRIVANDRUM MALE DHANUVACHAPURAM TRIVANDRUM 10 ABIJADAS A G GNANADHASON D Christian FEMALE VELLARADA, TRIVANDRUM 11 ABIN S SUNIL KUMAR C Christian MALE ELLUVILA 12 ABIN V M VIJAYAN N Christian TRIVANDRUM MALE KARAKONAM 13 AISHWARYA R RAJADAS N Christian FEMALE TRIVANDRUM KUNNATHUKAL 14 AKHILESH V A VINOD Y Christian MALE TRIVANDRUM KARAKKONAM 15 AKHIN A S AUSTIN T Christian MALE TRIVANDRUM KUNNATHUKAL 16 AKSHAY V P VINCENT R Christian TRIVANDRUM MALE PARASSALA 17 ALAN L SILVESTER LEEN Christian MALE TRIVANDRUM VELLARADA, 18 ALEENA S SAJI KUMAR S Christian FEMALE TRIVANDRUM PANACHAMOODU 19 ALSHAD.S SEYYAD ALI M Muslim MALE TRIVANDRUM ARAYOOR 20 ANCY A V VIJAYAKUMAR P Christian FEMALE TRIVANDRUM TRIVANDRUM STUDENTS LIST SL No Name Father's Name Community Address Gender ELLUVILA 21 ANCY MARY V S VINCENT M Christian FEMALE TRIVANDRUM VELLARADA 22 ANEENA V J VIJAYAKUMAR K Christian FEMALE TRIVANDRUM KOOTHALI 23 ANEESHA V LOWRANCE -

Voluntary Organizations.Pdf
1 List of voluntary organization with location of centre implementing Rajiv Gandhi National Creche Scheme - Kerala (Decentralized) Sl.N Name of the voluntary Full address of No. of units o. organization with full location of the centre sanctioned address 1 2 3 4 THIRUVANANTHAPURAM 1. F.No.CR-1/TVPM/2012-13/ R.C.Church 1 464 dt.08.08.2012 Edanji, Malayilkada, Manchavilakom.P.O, Anavoor Mahila Samajam Neyyattinkara Taluk, Kollayil Panchyath Anavoor, Perumkadavila, Thiruvananthapuram, Thiruvananthapuram 2. F.No.CR-2/TVPM/2012-13/ Aralummoodu, 1 464 dt. 08.08.2012 Trivandrum Dt. Aralummodu Vanitha Kshema Kendram, Aralummodu, Thiruvananthapuram. 3. F.No.CR-4/TVPM/2012-13/ 1. St.Joseph’s Church, 4 464 dt. 08.08.2012 Paliyode, Kottackal.P.O, Trivandrum. Baptist Memorial Mahila 2. St.Joseph’s Church, 2 Samajam, Paliyode, Kottackal.P.O, Trivandrum. Kottackal P.O., Perumkadavila, Thiruvananthapuram. 3. Holy Family Church, Chamavila, Kudayal.P.O, Trivandrum. 4. St.Mary’s Church, Thannikuzhy, Trivandrum. 3 4 F.No.CR-5/ TVPM/2012-13/ 1.Pulininnakattampotta, 464 dt. 08.08.2012 Kuttavila, Paraniyam, Poovar.P.O, Trivandrum. Bharath Social Service Centre, 7 Paraniyam, Poovar P.O. 2.Opp: to Church, Baby Thiruvananthapuram Creche, Karikkottakary, Kannur. 3.Near Milk Co- operative Society, Edapuzha, Iritty Block, Kannur. 4.BSSC Creche unit, Keezhpally, Kannur 5.Veerpad, Aralam Panchayath, Iritty Block, Kannur. 6.Madarsa L.P.School Aralam, Aralam Panchayath, Kannur. 7.Payam East, Iritty Block, Kannur. 5. F.No.CR-6/ TVPM/2012-13/ Near Milk Society, 1 464 dt. 08.08.2012 Kottukonam Road , 4 Velatharakonam, Bharatha Yathra Centre, Elluvila P.O. -
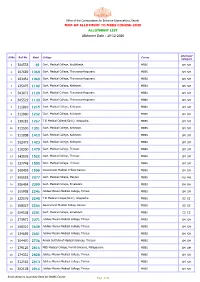
ALLOTMENT LIST MOP-UP ALLOTMENT to MBBS COURSE-2020 Allotment Date
Office of the Commissioner for Entrance Examinations, Kerala MOP-UP ALLOTMENT TO MBBS COURSE-2020 ALLOTMENT LIST Allotment Date : 20-12-2020 Allotment Sl.No. Roll No. Rank College Course Category 1 304533 39 Govt. Medical College, Kozhikkode. MBBS SM SM 2 107669 1068 Govt. Medical College, Thiruvananthapuram. MBBS SM SM 3 103451 1069 Govt. Medical College, Thiruvananthapuram. MBBS SM SM 4 135075 1102 Govt. Medical College, Kottayam. MBBS SM SM 5 303073 1128 Govt. Medical College, Thiruvananthapuram. MBBS SM SM 6 305529 1139 Govt. Medical College, Thiruvananthapuram. MBBS SM SM 7 111810 1215 Govt. Medical College, Kottayam. MBBS SM SM 8 312980 1232 Govt. Medical College, Kottayam. MBBS SM SM 9 130281 1267 T D Medical College(Govt.), Alappuzha. MBBS SM SM 10 311530 1351 Govt. Medical College, Kottayam. MBBS SM SM 11 311808 1410 Govt. Medical College, Kottayam. MBBS SM SM 12 302475 1423 Govt. Medical College, Kottayam. MBBS SM SM 13 110300 1479 Govt. Medical College, Thrissur. MBBS SM SM 14 143076 1522 Govt. Medical College, Thrissur. MBBS SM SM 15 137748 1590 Govt. Medical College, Thrissur. MBBS SM SM 16 300439 1599 Government Medical College Kannur MBBS SM SM 17 305518 2077 Govt. Medical College, Manjeri. MBBS MU MU 18 206454 2099 Govt. Medical College, Ernakulam MBBS BH BH 19 105958 2246 Jubilee Mission Medical College, Thrisur. MBBS SM SM 20 122576 2248 T D Medical College(Govt.), Alappuzha. MBBS EZ EZ 21 168027 2266 Government Medical College Kannur MBBS EZ EZ 22 304538 2281 Govt. Medical College, Ernakulam MBBS EZ EZ 23 173971 2371 Jubilee Mission Medical College, Thrisur. -

2014-15 Annul Report.Pdf
CENTRAL INSTITUTE OF CLASSICAL TAMIL (An autonomous Institution, Ministry of Human Resource Development, Department of Higher Education, Languages Division, Govt. of India) ANNUAL REPORT 2014 - 15 Central Institute of Classical TamilCentral >>>Institute Annualof Classical Tamil Report - Annual Report2014 2013 - 15 - 14 CENTRAL INSTITUTE OF CLASSICAL TAMIL Annual Report 2014 - 15 COMPOSITION Central Institute of Classical Tamil (CICT) was established at Chennai on 19th May 2008. CICT is registered as Society under section 10 of the Tamil Nadu Societies Registration Act, 1975 on 21st January 2009. ACTIVITIES OF THE INSTITUTE FOR THE YEAR 2014 – 15 he following list of forty-one ancient Tamil works belonging to the period up to A.D. 600 has been identified Tfor the purpose of studying the antiquity and uniqueness of ancient Tamils and their civilization. These classics have been specially selected for being exceptionally valuable and original compositions and they are not derivative or translated works. Apart from epigraphical and archaeological findings, these forty- one classics listed below are the only evidences that reflect the society, language and culture of the ancient Tamils. CentralCentral Institute Institute of Classical Tamil of - AnnualClassical Report 2013 Tamil - 14 >>> Annual Report 2014 - 15 11 Tolkāppiyam Eṭṭuttokai (Eight Anthologies) 1. Naṟṟiṇai 5. Paripāṭal 2. Kuṟuntokai 6. Kalittokai 3. Aiṅkuṟunūṟu 7. Akanāṉūṟu 4. Patiṟṟuppattu 8. Puṟanāṉūṟu Pattuppāṭṭu ( Ten Idylls) 1. Tirumurukāṟṟuppaṭai 6. Maturaikkāñci 2. Porunarāṟṟuppaṭai 7. Neṭunalvāṭai 3. Ciṟupāṇāṟṟuppaṭai 8. Kuṟiñcippāṭṭu 4. Perumpāṇāṟṟuppaṭai 9. Paṭṭiṉappālai 5. Mullaippāṭṭu 10.Malaipaṭukaṭām Patiṉeṇkīḻkkaṇakku 1. Nālaṭiyār 7. Aintiṇai Aimpatu 13.Tirukkuṟaḷ 2. Nāṇmaṇikkaṭikai 8. Aintiṇai Eḻupatu 14.Tirikaṭukam 3. Iṉṉā Nāṟpatu 9. Tiṇaimoḻi Aimpatu 15.Ācārakkōvai 4.