Original Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 COVID-19 Pandemic: Staged Management of Surgical Services
COVID-19 Pandemic: Staged Management of Surgical Services for Gynecology and Obstetrics Emily E. WEBER LEBRUN, MD, MS; Nash S. MOAWAD, MD, MS; E I. ROSENBERG, MD, Timothy E. MOREY, MD; Laurie Davies, MD; William O. COLLINS, MD; and John C. SMULIAN, MD, MPH Emily E. WEBER LEBRUN, MD, MS University of Florida College of Medicine, Department of Obstetrics & Gynecology Nash S. MOAWAD, MD, MS University of Florida College of Medicine, Department of Obstetrics & Gynecology Eric I. ROSENBERG, MD University of Florida College of Medicine, Department of Medicine Timothy E. MOREY, MD University of Florida College of Medicine, Department of Anesthesiology Laurie Davies, MD University of Florida College of Medicine, Department of Anesthesiology William O. COLLINS, MD University of Florida College of Medicine, Department of Otolaryngology John C. SMULIAN, MD, MPH University of Florida College of Medicine, Department of Obstetrics & Gynecology Acknowledgements: We would like to acknowledge the thoughtful contributions of Islam MD, Brian Hoh MD, Brent Carr MD, Jeffrey White MD, Laurie Davies MD and Parker Gibbs MD, all from the University of Florida College of Medicine without compensation. Disclosures: The authors have no relevant conflicts of interest or financial disclosures. Word Count: Abstract 168; Text 2043 1 Condensation: During the COVID-19 pandemic, a staged reduction in gynecologic and obstetric surgical services is needed to protect patients and the healthcare workforce, conserve personal protective equipment, and allow redeployment of facility resources. Short Title: Staged Surgical Postponement during COVID-19 Pandemic ABSTRACT: The COVID-19 pandemic has required an unprecedented global healthcare response requiring maintenance of existing hospital-based services while simultaneously preparing for high-acuity care for infected and sick individuals. -

Fungal Sinusitis Clinical Presentation, Latest Management: a Review Update
www.banglajol.info/index.php/JSF Journal of Science Foundation, January 2014, Vol. 12, No.1 ISSN 1728-7855 Review Article Fungal sinusitis clinical presentation, latest management: A Review Update Bithi Bhowmik1 Abstract Fungal sinusitis is a relatively common, often misdiagnosed disease process involving the paranasal sinuses. It is a serious condition, as certain forms of fungal sinusitis are associated with a high rate of mortality. Successful treatment requires a prompt diagnosis and frequently relies on radiologic imaging, specifically computed tomography (CT) and magnetic resonance (MR) imaging. The classification of fungal sinusitis is ever changing, but under the most current and widely accepted classification fungal sinusitis is broadly categorized as either invasive or noninvasive. Invasive fungal sinusitis is defined by the presence of fungal hyphae within the mucosa, submucosa, bone, or blood vessels of the paranasal sinuses. Invasive fungal sinusitis is subdivided into acute invasive fungal sinusitis, chronic invasive fungal sinusitis, and chronic granulomatous invasive fungal sinusitis. Conversely, noninvasive fungal sinusitis is defined by the absence of hyphae within the mucosal and other tissues of the paranasal sinuses. Noninvasive fungal sinusitis is subdivided into allergic fungal sinusitis and fungus ball (fungal mycetoma).[Journal of Science Foundation, 2014;12(1):16-19] Keywords: Fungal sinusitis; clinical presentation; management 1.0. Introduction Chronic sinusitis is a very common problem , and occurs even more frequently in people with allergies. A large number of people with chronic sinusitis are actually suffering from fungal sinus infections, which would not get better with typical antibiotics (Ferguson et al., 2000). Fungal infections of the sinuses have recently been blamed for causing most cases of chronic rhinosinusitis. -

Roll of Computed Tomography in Evaluation of NCCT Paranasal Sinuses
© 2020 JETIR April 2020, Volume 7, Issue 4 www.jetir.org (ISSN-2349-5162) Roll of Computed Tomography in Evaluation of NCCT Paranasal sinuses Ms. Nisha Jhinkwan,(1) , Mr.Binoo parihar,(2) Mr. Arshad alam khan,(3) Ms.Ashita Jain,(4) Ms.prachi Vishnoi,(5)Mr. Pradeep Bansal(6), Assistant Professor (Paramedical Department) Department of Radio Diagnosis, Imaging & Interventional Radiology. Abstract: Aim: this study aims to evaluate to accurate diagnose the diseases of paranasal sinuses. Subjects and Methods: All patients underwent Noncontrast CT (NCCT) of PNS on multi-detector CT Philips128 multi-slice unit. Result: A Maximum number of patients were in the age group of 16-30 years. The predominant chief presenting complaint was headache, followed by nasal discharged. The most common CT diagnosis was chronic sinusitis, mucosal thickening. Minimum patient’s diagnoses was polypoidal cyst and mass lesion. Conclusion: To conclude, this study proved good result of CT evaluation of diseases of paranasal sinuses. Introduction: Computed tomography (CT) of the sinuses uses special x-ray equipment to evaluate the paranasal sinus cavities – hollow, air-filled spaces within the bones of the face surrounding the nasal cavity. CT scanning is painless, noninvasive and accurate. It’s also the most reliable imaging technique for determining if the sinuses are obstructed and the best imaging modality for sinusitis. Computed tomography, more commonly known as a CT or CAT scan, is a diagnostic medical test that, like traditional x- rays, produces multiple images or pictures of the inside of the body. The cross-sectional images generated during a CT scan can be reformatted in multiple planes, and can even generate three-dimensional images. -

Fungal Sinusitis: a Case Report Daleen Jacobs CT Radiographer, Drs Nisbet, Govender & Assoc
Supplement Issue 2013 THE SOUTH AFRICAN RADIOGRAPHER peer reviewed CASE REPORT Fungal sinusitis: a case report Daleen Jacobs CT Radiographer, Drs Nisbet, Govender & Assoc. Inc., Richards Bay, South Africa Abstract This case report discusses fungal sinusitis in a healthy elderly woman. Her clinical history, radiological findings, the epidemiol- ogy and treatment options for fungal sinusitis are discussed. Keywords Aspergillosis, sphenoid sinuses, ethmoid sinuses, calcification, erosion, computed tomography. Case report requested an enhanced CT brain scan. vessels, bone, adjacent soft tissues, orbit [1] An elderly female patient consulted a This second CT scan included the maxil- and intracranial cavities . Early diagnosis neurosurgeon because of progressive lary sinuses. The findings were: opacifica- of fungal sinusitis may help prevent life- threatening complications in patients, headaches which were not responding to tion with calcification of the left sphenoid especially immunocompromised ones[2]. any form of treatment. She had a previous sinus as well as the posterior aspect of the ethmoid sinus; the left maxillary antrum The most common symptoms of fungal neck injury. The neurosurgeon requested demonstrated enhancement (Figure 4). No sinusitis are headaches, nasal obstruction a computed tomography (CT) scan of her other lesions were demonstrated. She was and purulent rhinorrhoea[3]. Patients can brain and a magnetic resonance imaging referred to an ear, nose and throat (ENT) however also present with an acute onset (MRI) scan of her cervical spine. specialist. She underwent endoscopic sur- of fever, epistaxis, sinus pain, cough, nasal In view of a possible allergy to iodine gery and a large amount of fungal debris mucosal ulcerations and crusting. -

Imaging for Rhinosinusitis
20, 2012 Health Technology Assessment Imaging for Rhinosinusitis Final Evidence Report April 3, 2015 Health Technology Assessment Program (HTA) Washington State Health Care Authority PO Box 42712 Olympia, WA 98504-2712 (360) 725-5126 hca.wa.gov/hta [email protected] Imaging for Rhinosinusitis A Health Technology Assessment Prepared for Washington State Healthcare Authority FINAL REPORT April 3, 2015 Acknowledgement This report was prepared by: Hayes, Inc. 157 S. Broad Street Suite 200 Lansdale, PA 19446 P: 215.855.0615 F: 215.855.5218 This report is intended to provide research assistance and general information only. It is not intended to be used as the sole basis for determining coverage policy or defining treatment protocols or medical modalities, nor should it be construed as providing medical advice regarding treatment of an individual’s specific case. Any decision regarding claims eligibility or benefits, or acquisition or use of a health technology is solely within the discretion of your organization. Hayes, Inc. assumes no responsibility or liability for such decisions. Hayes employees and contractors do not have material, professional, familial, or financial affiliations that create actual or potential conflicts of interest related to the preparation of this report. WA – Health Technology Assessment April 3, 2015 Table of Contents EXECUTIVE SUMMARY .................................................................................................................................. 1 Summary of Clinical Background ............................................................................................................. -

Fungal Rhinosinusitis Eng Cern Gan, Amin R Javer
CHAPTER 29 Fungal Rhinosinusitis Eng Cern Gan, Amin R Javer Snapshot Saprophytic Fungal Infection Acute (Fulminant) Invasive FRS Fungal Ball Granulomatous Invasive FRS Fungus-Related Eosinophilic FRS Chronic Invasive FRS Including Allergic Fungal Rhinosinusitis INTRODUCTION (3) fungus-related eosinophilic FRS, including allergic fun- gal rhinosinusitis (AFRS) or eosinphilic fungal rhinosinusitis Fungal rhinosinusitis (FRS) can be categorized into two (EFRS). Invasive FRS include (1) acute invasive (fulminant) 1 broad groups: (1) noninvasive and (2) invasive. This is FRS, (2) granulomatous invasive FRS and (3) chronic inva- based on the absence or presence of fungus in the tissue sive FRS.3 The clinical manifestations may overlap between (mucosa, blood vessel or bone) respectively.2 To avoid the different types of FRS and the disease may even progress confusion and to optimize management, the International from a noninvasive form to an invasive form with the change Society for Human and Animal Mycology group convened of immunologic status in a patient.4 Because fungus-related a panel of experts and published a consensus document in eosinophilic FRS forms the bulk of patients with FRS and 2009 on the categorization of FRS.3 Noninvasive conditions is laden with controversies in the pathogenesis, diagnosis include (1) saprophytic fungal infection, (2) fungal ball and and management, it will be the main focus of this chapter. NONINVASIVE FUNGAL RHINOSINUSITIS SAPROPHYTIC FUNGAL INFECTION Saprophytic fungal infection refers to visible fungal coloni- zation of mucus crusts seen within the nose and paranasal sinuses on nasoendoscopy3,4 (Fig. 1). These patients are usually asymptomatic or may present with a foul smelling odor.2 They are likely to have had previous sinus surgery. -

Invasive Fungal Sinusitis Invasive Fungal Sinusitis
AIJCR 10.5005/jp-journals-10013-1123 ARTICLE 2 Invasive Fungal Sinusitis Invasive Fungal Sinusitis Ashok K Gupta, Sandeep Bansal, Rijuneeta, Bhumika Gupta ABSTRACT increased frequency of these infections developed 4 Invasive sinus Aspergillus infection has been reported in the countries. last decade with increased frequency, most commonly in the Invasive and noninvasive syndromes of fungal sinusitis setting of hematologic malignancy, neutropenia, HIV infection share many features. They may occur in immunocompetent and other states of immunosuppression. Fungal rhinosinusitis or immunocompromised persons, may have an acute or can be broadly classified into two varieties—invasive and chronic course, and may extend beyond the thin walls of noninvasive on the basis of tissue invasion. Invasive fungal sinusitis are acute invasive, chronic invasive (both granulomatous the sinuses into the orbit, structures of the eye and the brain. and nongranulomatous forms), whereas noninvasive are fungus This fungal material is commonly associated with dense balls and allergic fungal sinusitis. Invasive fungal sinusitis is polyposis and calcification that results in areas of focal or one of the most challenging forms of sinonasal pathology to diffuse radiodensity on computed tomographic (CT) imaging manage, most commonly presenting in immunocompromised individuals. Chronic invasive being sinus aspergillosis (CISA) of the sinuses and decreased signal intensities on T1 and is being reported in immunocompetent patients at an increasing T2-weighted magnetic resonance imaging (MRI). Invasive rate while most of these cases are being reported from the India fungal sinusitis can be distinguished from noninvasive subcontinent and middle east. Invasive fungal sinusitis is on disease with the use of clinical criteria that include radiologic the rise worldwide and especially in north India as it is endemic in this part of the country. -

Surgical Management of Fungal Rhinosinusitis Surgical Management of Fungal Rhinosinusitis
REVIEW ARTICLE Surgical Management of Fungal Rhinosinusitis Surgical Management of Fungal Rhinosinusitis 1Bachi T Hathiram, 2Vicky S Khattar 1Professor and Head, Department of ENT and Head and Neck Surgery, TN Medical College and BYL Nair Charitable Hospital, Mumbai Maharashtra, India 2Assistant Professor, Department of ENT and Head and Neck Surgery, TN Medical College and BYL Nair Charitable Hospital, Mumbai Maharashtra, India Correspondence: Bachi T Hathiram, Professor and Head, Department of ENT and Head and Neck Surgery, TN Medical College and BYL Nair Charitable Hospital, MN Banaji Building, Flat No-2, Ground Floor, Forjett Street Cross Road Mumbai-400036, Maharashtra, India Abstract Fungal rhinosinusitis is on the rise. Most current treatment protocols for fungal rhinosinusitis include surgery combined with medical therapy. Endoscopic sinus surgery has revolutionized the management of this disease limiting the use of the open surgical approaches to very extensive cases with orbital, soft tissue or intracranial involvement by invasive fungal rhinosinusitis. A regular and thorough follow-up is mandatory in all cases to check for recurrences. This article discusses the various forms of fungal rhinosinusitis and their surgical management. Keywords: Fungal rhinosinusitis, fungus ball, invasive, endoscopic surgical procedures. INTRODUCTION anatomical areas such as the orbit or cranial cavity was frequently interpreted as invasive disease or malignancy and Since the etiologic agent and the host immunity influence radical surgeries followed resulting in increased morbity the choice of treatment, recent review of the treatment of and mortality in these cases. Treatment was generally fungal rhinosinusitis stresses the need for determining the aggressive, incorporating open surgical approaches as well type of disease: whether allergic, noninvasive or invasive. -

Medical Management of Allergic Fungal Rhinosinusitis Following Endoscopic Sinus Surgery: an Evidence‐Based Review An
REVIEW ARTICLE Medical management of allergic fungal rhinosinusitis following endoscopic sinus surgery: an evidence-based review and recommendations Eng Cern Gan, MBBS, MRCS (Edin), MMed (ORL), FAMS1, Andrew Thamboo, MHSc, MD1, Luke Rudmik, MD, MSc, FRCS (C)2, Peter H. Hwang, MD3, Berrylin J. Ferguson, MD, FACS, FAAOA4 and Amin R. Javer, BSc, MD, FRCSC, FARS1 Background: Allergic fungal rhinosinusitis (AFRS) is a sub- steroids; oral antifungals; topical antifungals; immunother- set of polypoid chronic rhinosinusitis that is characterized apy; and leukotriene modulators. by the presence of eosinophilic mucin with fungal hyphae within the sinuses and a TypeI hypersensitivity to fungi. The Conclusion: Based on available evidence in the literature, treatment of AFRS usually involves surgery in combination postoperative systemic and standard topical nasal steroids with medical therapies to keep the disease in a dormant are recommended in the medical management of AFRS. state. However, what constitutes an optimal medical regi- Nonstandard topical nasal steroids, oral antifungals, and im- men is still controversial. Hence, the purpose of this article munotherapy are options in cases of refractory AFRS. No is to provide an evidence-based approach for the medical recommendations can be provided for topical antifungals management of AFRS. and leukotriene modulators due to insufficient clinical re- search reported in the literature. C 2014 ARS-AAOA, LLC. Methods: A systemic review of the literature on the med- ical management of AFRS was performed using Medline, Key Words: EMBASE, and Cochrane Review Databases up to March 15, allergic fungal rhinosinusitis; AFRS; evidence-based 2013. The inclusion criteria were as follows: patients >18 medicine; medical management; endoscopic sinus surgery years old; AFRS as defined by Bent and Kuhn; post–sinus surgery; studies with a clearly defined end point to evalu- ate the effectiveness of medical therapy in postoperative HowtoCitethisArticle: Gan EC, Thamboo A, Rudmik L, AFRS patients. -
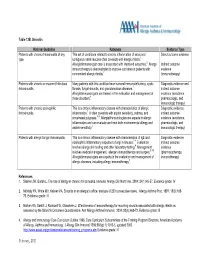
Table 13B Sinusitis
Table 13B. Sinusitis Referral Guideline Rationale Evidence Type Patients with chronic rhinosinusitis of any This set of conditions related to chronic inflammation of sinus and Direct outcome evidence type. contiguous nasal mucosa often co-exists with allergic rhinitis. 1 Allergist/immunologist care is associated with improved outcomes. 2 Allergy Indirect outcome immunotherapy is demonstrated to improve outcomes in patients with evidence concomitant allergic rhinitis.3 (immunotherapy) Patients with chronic or recurrent Infectious Many patients with this condition have humoral immunodeficiency, cystic Diagnostic evidence and rhinosinusitis. fibrosis, fungal sinusitis, and granulomatous diseases. 1 indirect outcome Allergist/immunologists are trained in the evaluation and management of evidence (avoidance, these disorders. 4 pharmacologic, and immunologic therapy) Patients with chronic eosinophilic This is a chronic inflammatory disease with characteristics of allergic Diagnostic evidence, rhinosinusitis. inflammation. 1 It often co-exists with aspirin sensitivity, asthma, and indirect outcome sinus/nasal polyposis. 5, 6 Allergist/immunologists are experts in allergic evidence (avoidance, inflammation and can evaluate and treat both environmental allergy and pharmacologic, and aspirin sensitivity. 4 immunologic therapy) Patients with allergic fungal rhinosinusitis. This is a chronic inflammatory disease with characteristics of IgE and Diagnostic evidence, eosinophilic inflammatory response to fungi in sinuses. 7, 8 Evaluation indirect outcome involves allergy skin testing and other laboratory testing. 9 Management evidence involves medical management, allergen immunotherapy and surgery. 9, 10 (pharmacotherapy, Allergist/immunologists are experts in the evaluation and management of immunotherapy) allergic diseases, including allergy immunotherapy.4 References: 1. Steinke JW, Borish L. The role of allergy in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2004; 24(1):45-57. -

Fungal Infections in the Intensive Care Unit GRADUATE THESIS
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Veterinary medicine - Repository of PHD, master's thesis UNIVERSITY OF ZAGREB SCHOOL OF MEDICINE Dajana Džeko Fungal Infections in the Intensive Care Unit GRADUATE THESIS Zagreb, 2017 This graduation paper has been done at the Department of Anaesthesiology at the University of Zagreb under the supervision of Dr. Sc. Tajana Zah Bogović during the academic year 2016 /2017. Table of Contents SUMMARY ............................................................................................................................... 1 SAŽETAK ................................................................................................................................. 2 1. INTRODUCTION.................................................................................................................. 3 2. EPIDEMIOLOGY OF FUNGAL INFECTIONS IN THE ICU ............................................... 4 2.1 Candida Infections ................................................................................................................... 4 2.2 Non-Candida Infections .......................................................................................................... 5 3. RISK FACTORS FOR FUNGAL INFECTIONS IN THE ICU ............................................. 6 4. DIAGNOSIS OF FUNGAL INFECTIONS ......................................................................... 11 4.1 Traditional Methods .............................................................................................................. -

The Diagnosis and Management of Sinusitis: a Practice Parameter Update
The diagnosis and management of sinusitis: A practice parameter update Chief Editors: Raymond G. Slavin, MD, Sheldon L. Spector, MD, and I. Leonard Bernstein, MD Sinusitis Update Workgroup: Chairman—Raymond G. Slavin, MD; Members—Michael A. Kaliner, MD, David W. Kennedy, MD, Frank S. Virant, MD, and Ellen R. Wald, MD Joint Task Force Reviewers: David A. Khan, MD, Joann Blessing-Moore, MD, David M. Lang, MD, Richard A. Nicklas, MD,* John J. Oppenheimer, MD, Jay M. Portnoy, MD, Diane E. Schuller, MD, and Stephen A. Tilles, MD Reviewers: Larry Borish, MD, Robert A. Nathan, MD, Brian A. Smart, MD, and Mark L. Vandewalker, MD These parameters were developed by the Joint Task Force on Practice participants, no single individual, including those who served on Parameters, representing the American Academy of Allergy, Asthma the Joint Task force, is authorized to provide an official AAAAI or and Immunology; the American College of Allergy, Asthma and ACAAI interpretation of these practice parameters. Any request for Immunology; and the Joint Council of Allergy, Asthma and information about or an interpretation of these practice parameters Immunology. by the AAAAI or the ACAAI should be directed to the Executive Offices of the AAAAI, the ACAAI, and the Joint Council of Allergy, The American Academy of Allergy, Asthma and Immunology (AAAAI) Asthma and Immunology. These parameters are not designed for and the American College of Allergy, Asthma and Immunology use by pharmaceutical companies in drug promotion. (ACAAI) have jointly accepted responsibility for establishing ‘‘The diagnosis and management of sinusitis: a practice parameter Published practice parameters of the Joint Task Force update.’’ This is a complete and comprehensive document at the current time.