State of Wildlife in the Jackson Hole Area
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IMBCR Report
Integrated Monitoring in Bird Conservation Regions (IMBCR): 2015 Field Season Report June 2016 Bird Conservancy of the Rockies 14500 Lark Bunting Lane Brighton, CO 80603 303-659-4348 www.birdconservancy.org Tech. Report # SC-IMBCR-06 Bird Conservancy of the Rockies Connecting people, birds and land Mission: Conserving birds and their habitats through science, education and land stewardship Vision: Native bird populations are sustained in healthy ecosystems Bird Conservancy of the Rockies conserves birds and their habitats through an integrated approach of science, education and land stewardship. Our work radiates from the Rockies to the Great Plains, Mexico and beyond. Our mission is advanced through sound science, achieved through empowering people, realized through stewardship and sustained through partnerships. Together, we are improving native bird populations, the land and the lives of people. Core Values: 1. Science provides the foundation for effective bird conservation. 2. Education is critical to the success of bird conservation. 3. Stewardship of birds and their habitats is a shared responsibility. Goals: 1. Guide conservation action where it is needed most by conducting scientifically rigorous monitoring and research on birds and their habitats within the context of their full annual cycle. 2. Inspire conservation action in people by developing relationships through community outreach and science-based, experiential education programs. 3. Contribute to bird population viability and help sustain working lands by partnering with landowners and managers to enhance wildlife habitat. 4. Promote conservation and inform land management decisions by disseminating scientific knowledge and developing tools and recommendations. Suggested Citation: White, C. M., M. F. McLaren, N. J. -

Grand Teton National Park News Release
National Park Service Grand Teton PO Box 170 U.S. Department of the Interior National Park Moose, Wyoming 83012 FOR IMMEDIATE RELEASE Jackie Skaggs/307.739.3393 January 08, 2010 10-01 Grand Teton National Park News Release Environmental Assessment Available for Public Review on Site Work for Grand Teton National Park Headquarters Rehabilitation Project Grand Teton National Park Superintendent Mary Gibson Scott announced today that the Moose Headquarters Rehabilitation Site Work Environmental Assessment (EA) is now available for public review. This EA will be open to review for 30 days, from January 11 through February 9, 2010. The National Park Service (NPS) proposes to perform site improvements that are designed to enhance visitor services and address employee health and safety deficiencies at Grand Teton National Park’s headquarters area in Moose, Wyoming. The site work would restructure vehicle/pedestrian access points, promote better traffic flow, reduce user-created trails and consolidate pedestrian walkways, and improve way-finding throughout the Moose headquarters complex. The purpose of the proposal is to upgrade and improve conditions in a way that enhances visitors’ experiences while providing a safe, healthy, and functional working/living environment for park employees and their families. The NPS preferred alternative involves the reconfiguration of vehicle and pedestrian traffic within the park administrative area and the Moose river landing access, the removal of several temporary buildings, and restoration work targeted at providing appropriate stormwater management. The proposed improvements are designed to increase visitor and employee safety, refine parking and traffic flow patterns, reduce the built environment, and improve water quality while still preserving the character of the area and protecting natural and cultural resources. -

WYOMING Adventure Guide from YELLOWSTONE NATIONAL PARK to WILD WEST EXPERIENCES
WYOMING adventure guide FROM YELLOWSTONE NATIONAL PARK TO WILD WEST EXPERIENCES TravelWyoming.com/uk • VisitTheUsa.co.uk/state/wyoming • +1 307-777-7777 WIND RIVER COUNTRY South of Yellowstone National Park is Wind River Country, famous for rodeos, cowboys, dude ranches, social powwows and home to the Eastern Shoshone and Northern Arapaho Indian tribes. You’ll find room to breathe in this playground to hike, rock climb, fish, mountain bike and see wildlife. Explore two mountain ranges and scenic byways. WindRiver.org CARBON COUNTY Go snowmobiling and cross-country skiing or explore scenic drives through mountains and prairies, keeping an eye out for foxes, coyotes, antelope and bald eagles. In Rawlins, take a guided tour of the Wyoming Frontier Prison and Museum, a popular Old West attraction. In the quiet town of Saratoga, soak in famous mineral hot springs. WyomingCarbonCounty.com CODY/YELLOWSTONE COUNTRY Visit the home of Buffalo Bill, an American icon, at the eastern gateway to Yellowstone National Park. See wildlife including bears, wolves and bison. Discover the Wild West at rodeos and gunfight reenactments. Hike through the stunning Absaroka Mountains, ride a mountain bike on the “Twisted Sister” trail and go flyfishing in the Shoshone River. YellowstoneCountry.org THE WORT HOTEL A landmark on the National Register of Historic Places, The Wort Hotel represents the Western heritage of Jackson Hole and its downtown location makes it an easy walk to shops, galleries and restaurants. Awarded Forbes Travel Guide Four-Star Award and Condé Nast Readers’ Choice Award. WortHotel.com welcome to Wyoming Lovell YELLOWSTONE Powell Sheridan BLACK TO YELLOW REGION REGION Cody Greybull Bu alo Gillette 90 90 Worland Newcastle 25 Travel Tips Thermopolis Jackson PARK TO PARK GETTING TO KNOW WYOMING REGION The rugged Rocky Mountains meet the vast Riverton Glenrock Lander High Plains (high-elevation prairie) in Casper Douglas SALT TO STONE Wyoming, which encompasses 253,348 REGION ROCKIES TO TETONS square kilometres in the western United 25 REGION States. -

1 KECK PROPOSAL: Eocene Tectonic Evolution of the Teton-Absaroka
KECK PROPOSAL: Eocene Tectonic Evolution of the Teton-Absaroka Ranges, Wyoming (Year 2) Project Leaders: John Craddock (Macalester College; [email protected]) and Dave Malone (Illinois State University; [email protected]) Host Institution: Macalester College, St. Paul, MN Project Dates: ~July 15-August 14, 2011 Student Prerequisites: Structural Geology, Sedimentology. Preamble: This project is an expansion of a 2010 Keck project that was funded at a reduced level (Craddock, 3 students); Malone and 4 students participated with separate funding. We completed or are currently working on three 2010 projects: 1. Structure, geochemistry and geochronology (U-Pb zircon) of carbonate pseudotachylite injection, White Mtn. (J. Geary, Macalester; note that this was not part of last year’s proposal but a new discovery in 2010 caused us to redirect our efforts), 2. Calcite twinning strains within the S. Fork detachment allochthon, northwest, WY (K. Kravitz, Smith; note because of a heavy snow pack in the Tetons this past summer, we chose a different structure to study), and 3. Provenance of heavy minerals and detrital zircon geochronology, Eocene Absaroka volcanics, northwest, WY (R. McGaughey, Carleton). We did not sample the footwall folds proposed in the previous proposal (under snow) and will focus on this project and mapping efforts of White Mountain and the 40 x 10 km S. Fork detachment area near Cody, WY, in part depending on the results (calcite strains, detrital zircons) of the 2010-11 effort. All seven students are working on the detrital zircon geochronology project, and two abstracts are accepted at the 2011 Denver GSA meeting. Overview: This proposal requests funding for 2 faculty to engage 6 students researching a variety of outstanding problems in the tectonic evolution of the Sevier-Laramide orogens as exposed in the Teton and Absaroka ranges in northwest Wyoming. -

National Monuments and the Forest Service
NATIONAL MONUMENTS AND THE FOREST SERVICE Gerald W. Williams, Ph.D., (Retired) USDA Forest Service Washington, DC National monuments are areas of federal land set aside by the Congress or most often by the president, under authority of the American Antiquities Act of June 8, 1906, to protect or enhance prominent or important features of the national landscape. Such important national features include those land areas that have historic cultural importance (sites and landmarks), prehistoric prominence, or those of scientific or ecological significance. Today, depending on how one counts, there are 81 national monuments administered by the USDI National Park Service, 13 more administered by the USDI Bureau of Land Management (BLM), five others administered by the USDA Forest Service, two jointly managed by the BLM and the National Park Service, one jointly administered by the BLM and the Forest Service, one by the USDI Fish & Wildlife Service, and another by the Soldiers’ and Airmen’s Home in Washington, D.C. In addition, one national monument is under National Park Service jurisdiction, but managed by the Forest Service while another is on USDI Bureau of Reclamation administered land, but managed by the Park Service. The story of the national monuments and the Forest Service also needs to cover briefly the creation of national parks from national forest and BLM lands. More new national monuments and national parks are under consideration for establishment. ANTIQUITIES ACT OF 1906 Shortly after the turn of the century, many citizens’ groups and organizations, as well as members of Congress, believed it was necessary that an act of Congress be passed to combat the increasing acts of vandalism and even destruction of important cultural (historic and prehistoric), scenic, physical, animal, and plant areas around the country (Rothman 1989). -

National Forest Imagery Catalog Collection at the USDA
National Forest Imagery Catalog collection at the USDA - Farm Service Agency Aerial Photography Field Office (APFO) 2222 West 2300 South Salt Lake City, UT 84119-2020 (801) 844-2922 - Customer Service Section (801) 956-3653 - Fax (801) 956-3654 - TDD [email protected] http://www.apfo.usda.gov This catalog listing shows the various photographic coverages used by the U.S. Department of Agriculture and archived at the Aerial Photography Field Office. This catalog references U.S. Forest Service (FS) and other agencies imagery. For imagery prior to 1955, please contact the National Archives & Records Administration: Cartographic & Architectural Reference (NWCS-Cartographic) Aerial Photographs Team http://www.archives.gov/research/order/maps.html#contact Coverage of U.S. Forest Service photography is listed alphabetically for each forest within a region. Numeric and alpha codes used to identify FS projects are determined by the Forest Service. The original film type for most of this imagery is a natural color negative. Line indexes are available for most projects. The number of index sheets required to cover a project area is shown on the listing. Please reference the remarks column, which may identify a larger or smaller project area than the National Forest area defined in the header. Offered in the catalog listing at each National Forest heading is a link to locate the Regional and National Forest office address and phone number at: http://www.fs.fed.us/intro/directory You may wish to visit the National Forest office to view the current imagery and have them assist you in identifying aerial imagery from the APFO. -

Bridger-Teton National Forest Evaluation of Areas with Wilderness Potential
BTNF Evaluation of Areas with Wilderness Potential 2008 BRIDGER-TETON NATIONAL FOREST EVALUATION OF AREAS WITH WILDERNESS POTENTIAL Phillips Ridge Roadless Area 9/23/2009 1 CONTENTS Introduction ..................................................................................................................................2 The 2001 roadless rule, areas with wilderness potential, and process for integration .................2 Capability factors defined ............................................................................................................4 Availability defined .....................................................................................................................9 Need defined ................................................................................................................................9 BTNF areas with wilderness potential .........................................................................................11 Eligibility factors by area .............................................................................................................15 Summary of capability factors .....................................................................................................68 Areas with Wilderness potential and Forest Plan revision ..........................................................70 INTRODUCTION Roadless areas were identified during the Roadless Area Review and Evaluation II (RARE II) analysis conducted in 1978 and re-evaluated in 1983 to include all areas of at least -

Uppermost Cretaceous and Tertiary Stratigraphy of Fossil Basin, Southwestern Wyoming
Uppermost Cretaceous and Tertiary Stratigraphy of Fossil Basin, Southwestern Wyoming By STEVEN S. ORIEL and JOSHUA I. TRACEY, JR. GEOLOGICAL SURVEY PROFESSIONAL PAPER 635 New subdivisions of the J,ooo-Joot-thick continental Evanston, Wasatch, Green River, and Fowkes Formations facilitate understanding of sediment genesis and Jl7yoming thrust-belt tectonic events UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON 1970 UNITED STATES DEPARTMENT OF THE INTERIOR WALTER J. HICKEL, Secretary GEOLOGICAL SURVEY William T. Pecora, Director Library of Congress catalog-card No. 70-604646 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price 65 cents (paper cover) CONTENTS Page Wasatch Formation-Continued Abstract __________________________________________ _ 1 Fossils and age-Continued· Page Introduction ______________________________________ _ 2 Tunp Member______________________________ 28 Purpose ______________________________________ _ 2 Origin--------~-------------------------------- 28 Earlier work_ .. __ - __ - ___________________ - _-- _- __ 2 Tectonic implications ____________ -_-------------- 29 Acknowledgments __ . ___________________________ _ 2 Green River Formation ___ .. _______ ------------------ 30 General relations ___ -- _________________________ _ 5 Name and usage __________________ -------------- 30 Evanston Formation _______________________________ _ 5 Definition __________________ -_-------------- 30 N arne and usage _______________________________ _ 5 Lithologic heterogeneity. -

Exploring Grand Teton National Park
05 542850 Ch05.qxd 1/26/04 9:25 AM Page 107 5 Exploring Grand Teton National Park Although Grand Teton National Park is much smaller than Yel- lowstone, there is much more to it than just its peaks, a dozen of which climb to elevations greater than 12,000 feet. The park’s size— 54 miles long, from north to south—allows visitors to get a good look at the highlights in a day or two. But you’d be missing a great deal: the beautiful views from its trails, an exciting float on the Snake River, the watersports paradise that is Jackson Lake. Whether your trip is half a day or 2 weeks, the park’s proximity to the town of Jackson allows for an interesting trip that combines the outdoors with the urbane. You can descend Grand Teton and be living it up at the Million Dollar Cowboy Bar or dining in a fine restaurant that evening. The next day, you can return to the peace of the park without much effort at all. 1 Essentials ACCESS/ENTRY POINTS Grand Teton National Park runs along a north-south axis, bordered on the west by the omnipresent Teton Range. Teton Park Road, the primary thoroughfare, skirts along the lakes at the mountains’ base. From the north, you can enter the park from Yellowstone National Park, which is linked to Grand Teton by the John D. Rockefeller Jr. Memorial Parkway (U.S. Hwy. 89/191/287), an 8-mile stretch of highway, along which you might see wildlife through the trees, some still bare and black- ened from the 1988 fires. -

Eocene Green River Formation, Western United States
Synoptic reconstruction of a major ancient lake system: Eocene Green River Formation, western United States M. Elliot Smith* Alan R. Carroll Brad S. Singer Department of Geology and Geophysics, University of Wisconsin, 1215 West Dayton Street, Madison, Wisconsin 53706, USA ABSTRACT Members. Sediment accumulation patterns than being confi ned to a single episode of arid thus refl ect basin-center–focused accumula- climate. Evaporative terminal sinks were Numerous 40Ar/39Ar experiments on sani- tion rates when the basin was underfi lled, initially located in the Greater Green River dine and biotite from 22 ash beds and 3 and supply-limited accumulation when the and Piceance Creek Basins (51.3–48.9 Ma), volcaniclastic sand beds from the Greater basin was balanced fi lled to overfi lled. Sedi- then gradually migrated southward to the Green River, Piceance Creek, and Uinta ment accumulation in the Uinta Basin, at Uinta Basin (47.1–45.2 Ma). This history is Basins of Wyoming, Colorado, and Utah Indian Canyon, Utah, was relatively con- likely related to progressive southward con- constrain ~8 m.y. of the Eocene Epoch. Mul- stant at ~150 mm/k.y. during deposition of struction of the Absaroka Volcanic Prov- tiple analyses were conducted per sample over 5 m.y. of both evaporative and fl uctuat- ince, which constituted a major topographic using laser fusion and incremental heating ing profundal facies, which likely refl ects the and thermal anomaly that contributed to a techniques to differentiate inheritance, 40Ar basin-margin position of the measured sec- regional north to south hydrologic gradient. loss, and 39Ar recoil. -
Annual Report
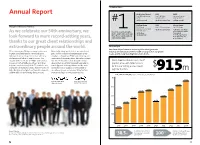
Top Ranking Report Annual Report Architectural Record ENR VMSD Top 300 Architecture Top 150 Global Top Retail Design Firms: Design Firms: Firms of 2014: # #1 Firm Overall #1 Architecture Firm #1 Firm Overall Building Design ENR Interior Design Message from the Board of Directors 2014 World Top 500 Design Firms: Top 100 Giants: Architecture 100 Most #1 Architecture Firm #1 Architecture Firm Admired Firms: Gensler is1 a leader among the #1 in Corporate Office As we celebrate our 50th anniversary, we world’s architecture and design #1 US Firm #1 in Retail #4 Global Firm #1 in Transportation firms. Here’s how we ranked in #1 in Government look forward to more record-setting years, our industry in 2014. #1 in Cultural thanks to our great client relationships and extraordinary people around the world. Financial Report Our financial performance and recognition throughout the We’re entering our 50th year stronger than ever. Financially strong and debt-free, we contributed industry are indications of the breadth of our practice, our global In 2014, our global growth continued apace $38.5 million in deferred compensation to our reach, and the long-standing trust of our clients. with our clients as they entrusted us with new employees through our ESOP, profit-sharing, and challenges and led us to new locations. Our international retirement plans. We made strategic expanded Gensler team of 4,700+ professionals investments in our research and professional We’ve broadened our services to 27 now work from 46 different offices. With their development programs, along with upgrades to practice areas, with total revenues help, we completed projects in 72 countries and our design-and-delivery platform and the tools for the year setting a new record $ increased our revenues to $915 million—a record and technology to support it. -

Membership List April 2021
Membership List April 2021 Albuquerque International Sunport (ABQ) Fairbanks Int’l. Airport (FAI) Allegheny County Airport Authority (PIT) Fresno Yosemite International Airport (FAT) Austin-Bergstrom Int’l. Airport (AUS) AvPorts-Westchester County Airport (HPN) George Bush Intercontinental Airport (IAH) Greater Asheville Regional Airport Auth. (AVL) Bangor International Airport (BGR) Greater Orlando Aviation Authority (MCO) Barnstable Municipal Airport (HYA) Greater Rockford Airport Authority (RFD) Bishop International Airport Authority (FNT) Greenville/Spartanburg Int’l. Airport (GSP) Blue Grass Airport (LEX) Gulfport-Biloxi International Airport (GPT) Boise Airport (BOI) Broward County Aviation Dept. (FLL) Hagerstown Regional Airport (HGR) Buffalo Niagara Int’l. Airport (BUF) Hartsfield-Atlanta International Airport (ATL) Houston Airport System (EFD, HOU, IAH) Calgary Airport Authority (YYC) Huntsville-Madison Cnty. Airport Auth. (HSV) Charles M. Shulz-Sonoma County Airport (STS) Chattanooga Metropolitan Airport Auth. (CHA) Islip MacArthur Airport (ISP) Chicago Rockford Int’l. Airport (RFD) Cincinnati/No. Kentucky Int’l. Airport (CVG) Jackson Hole Airport Board (JAC) City of Chicago Aeronautics Dept. (ORD) Jackson Municipal Airport Authority (JAN) City of Dallas, Dallas Love Field (DAL) Jacksonville Aviation Authority (JAX) City of Redding Airports Division (RDD) John Wayne Airport—Orange County (SNA) Cleveland Hopkins Int’l. Airport (CLE) Colorado Springs Airport (COS) Kansas City International Airport (MCI) Columbus Regional Airport